This set of Class 12 Chemistry Chapter 10 Multiple Choice Questions & Answers (MCQs) focuses on “Preparation of Haloarenes”.
1. Which of the following cannot be a catalyst in the electrophilic substitution of arenes?
a) FeCl3
b) FeBr3
c) NH3
d) AlCl3
View Answer
Explanation: The electrophilic substitution of arenes requires the presence of a Lewis acid catalyst which acts as a halogen carrier. NH3 is a Lewis base.
2. Which of the following are the most suitable conditions for electrophilic substitution of arenes?
a) UV light and heat
b) Cold and dark
c) 40oC temperature and dark
d) Room temperature and sunlight
View Answer
Explanation: This reaction is carried out in the dark at ordinary temperatures (310-320K) in the presence of a Lewis acid catalyst.
3. When excess chlorine is used in electrophilic substitution of toluene, which of the following dichloroarenes are formed?
a) ortho and meta
b) ortho and para
c) meta and para
d) ortho, meta and para
View Answer
Explanation: When excess halogen is used, the second halogen also gets incorporated into the aromatic ring at ortho and para positions with respect to the first halogen. This is because the CH3 group in toluene is ortho and para directing.
4. The ortho and para isomers formed by electrophilic substitution of arenes can be easily separated due to the large difference in their ________
a) densities
b) solubilities
c) melting points
d) boiling points
View Answer
Explanation: The solid form of ortho isomer melts way earlier than the para isomer as the latter has a higher melting point due to its symmetric geometry.
5. In the iodination of benzene, which compound causes the reduction of iodobenzene back to benzene?
a) Iodic acid
b) Hydrogen iodide
c) Nitric acid
d) Mercuric oxide
View Answer
Explanation: The hydrogen iodide formed during the iodination of benzene is strong reducing agent which reverses the reaction. To prevent this, oxidising agents like HIO4, HNO3 and HgO are used in the reaction so as to oxidize the HI to iodine.
6. What is the most suitable temperature for the formation of a diazonium salt from aniline?
a) 0°C
b) 18°C
c) 37°C
d) 60°C
View Answer
Explanation: Diazonium salts are formed by treating ice-cold solution of aromatic amines in aqueous mineral acid with sodium nitrite at low temperatures of 273-278K.
7. What is the by-product formed along with the haloarene at the end of Sandmeyer’s reaction?
a) H2O
b) HCl
c) NO2
d) N2
View Answer
Explanation: When a diazonium salt solution is treated with cuprous chloride/bromide, it gives an aryl halide along with nitrogen as. This is called Sandmeyer’s reaction.
8. Identify the missing compound Y.
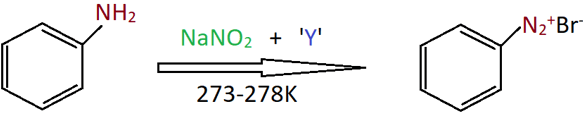
a) NaBr
b) HBr
c) HCl
d) Cu2Br2
View Answer
Explanation: This is the diazotisation reaction where an aromatic amine in aqueous mineral acid (HBr) is treated with NaNO2 at cold temperature to form respective diazonium salts.
9. Sandmeyer’s reaction can be used to prepare fluoroarenes.
a) True
b) False
View Answer
Explanation: Sandmeyer’s reaction is used only for preparing alkyl chlorides and bromides. Fluoroarenes are prepared by a different method called Balz-Schiemann reaction.
10. Which of the following is a reagent used in the preparation of benzene diazonium chloride from aniline?
a) Sodium hydroxide
b) Sodium chloride
c) Sodium nitrite
d) Sodium nitrate
View Answer
Explanation: Sodium nitrite reacts with aqueous HCl to form NaCl and HNO2. The nitrous acid reacts with aniline in cold aqueous solution of HCl to form benzene diazonium chloride.
11. What happens when a solution of benzene diazonium bromide and an aqueous solution potassium iodide are shaken together?
a) Iodobenzene is formed
b) Bromobenzene is formed
c) A dihaloarene is formed
d) No reaction
View Answer
Explanation: Since iodine is more reactive, the replacement of diazonium group to form iodobenzene does not require the presence of a cuprous halide and is done simply by shaking the diazonium salt with KI.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Mathematics MCQs
