This set of Class 12 Chemistry Chapter 11 Multiple Choice Questions & Answers (MCQs) focuses on “Alcohols and Phenols – 3”.
1. When the bond between O and H of the hydroxyl group is broken, alcohols react as _________
a) nucleophiles
b) electrophile
c) protonated molecules
d) electron seeking compounds
View Answer
Explanation: Alcohols may react as nucleophiles or electrophiles depending upon whether the O-H bond or the C-O bond is broken respectively. Carbocations are formed in the nucleophile case and protonated alcohols are formed in the electrophile case.
2. Alcohols and phenols are ________
a) Lewis acids
b) Lewis bases
c) Bronsted acids
d) Bronsted bases
View Answer
Explanation: Alcohols and phenols are known to be acidic in nature based on their interaction with metals. They are able to donate a proton to a stronger base. Additionally, alcohols also act as Bronsted bases due to the presence of unshared electron pairs on oxygen.
3. What is the correct relation between acidic strength of primary, secondary and tertiary alcohols?
a) 1°>2°>3°
b) 1°<2°<3°
c) 1°=2°=3°
d) 1°>3°>2°
View Answer
Explanation: Alkyl groups are electron releasing groups and tend to increase the electron density on oxygen and reduce the polarity of O-H bond. So, the greater the number of alkyl groups present, lesser will be the tendency to release protons, and therefore weaker will be the acidic strength.
4. Water is a ______ than alcohol.
a) better proton acceptor
b) stronger base
c) weaker acid
d) better proton donor
View Answer
Explanation: The reaction between water and an alkoxide illustrates that water is a better proton donor (stronger acid) than alcohol.
5. Phenols are stronger acids than alcohols.
a) True
b) False
View Answer
Explanation: The aryl carbon in phenols is more electronegative than the sp3 hybridized carbon in alcohols due to the presence of double bond. This extra negative charge is also delocalized at positions across the benzene, resulting in resonating structure and hence stability.
6. Which of the following compounds is the best proton acceptor?
a) Ethanol
b) Phenol
c) p-Cresol
d) p-Nitrophenol
View Answer
Explanation: Proton acceptor indicates the basic nature of the compound. Ethanol is the only alcohol in the given compounds, and it is the least acidic, because phenols are more acidic than alcohols due to their stability through resonance structures.
7. Which of the following phenols is the most acidic?
a) o-Cresol
b) m-Cresol
c) o-Nitrophenol
d) m-Nitrophenol
View Answer
Explanation: Alkyl groups are electron releasing, and contribute towards a reduction in acidic strength of phenols. Electron withdrawing groups like nitro, enhances the acidic strength especially at ortho and para positions, due to effective delocalisation of negative charge in the phenoxide ion.
8. What is the role of concentrated H2SO4 in the reaction between alcohol and carboxylic acid?
a) Deprotonating agent
b) Dehydrating agent
c) Reducing agent
d) Nucleophile
View Answer
Explanation: Concentrated H2SO4 is the catalyst in the esterification of alcohols, which acts a protonating and dehydrating agent. The reaction is reversible and is shifted in the forward direction by the removal of water as soon as it is formed.
9. Which of the following alcohols is the most reactive towards esterification reaction?
a) CH3OH
b) CH3CH2OH
c) (CH3)2CHOH
d) (CH3)3COH
View Answer
Explanation: Presence of bulky groups reduces the reactivity of alcohols and carboxylic acid towards esterification due to effect of stearic hinderance. Therefore, the tertiary alcohols are least reactive.
10. Identify the products of the following reaction:
Methyl alcohol + Ethyl magnesium bromide = ?
a) CH4 and CH3OMgBr
b) CH4 and CH3CH2OMgBr
c) C2H6 and CH3OMgBr
d) C2H6 and CH3CH2OMgBr
View Answer
Explanation: Alcohols reacts with Grignard reagents to form hydrocarbons. Also, the alkane formed from this reaction corresponds to the alkyl group of the Grignard reagent and not the alcohol.
11. What is the catalyst used in the acetylation of alcohols?
a) Palladium
b) Hydrogen peroxide
c) Pyridine
d) Aluminium chloride
View Answer
Explanation: The reaction of alcohols with acid chlorides and acid anhydride is carried out in the presence of a base so as to neutralise the acid formed during the reaction. This facilitates the shift f equilibrium in the forward direction.
12. Aspirin is produced from the reaction between salicylic acid and which compound?
a) Acetyl chloride
b) Acetic anhydride
c) Phenyl acetate
d) Benzoyl chloride
View Answer
Explanation: Salicylic acid, which is a phenolic acid when treated with acetic anhydride produces acetyl salicylic acid, also known as aspirin.
13. What is the correct order of reactivity of alcohols toward reactions involving cleavage of C-O bond?
a) 1°>2°>3°
b) 3°>2°>1°
c) 1°>3°>2°
d) 3°>1°>2°
View Answer
Explanation: Alkyl groups are electron releasing groups and increase the electron density towards oxygen making the C-O more polar. This makes the cleavage of the bond between C and O a lot easier.
14. What is the catalyst used in the following reaction?
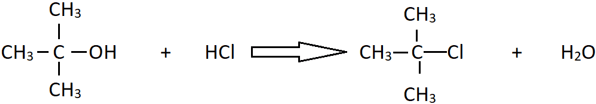
a) Anhydrous AlCl3
b) Anhydrous FeCl3
c) Anhydrous ZnCl2
d) No catalyst required
View Answer
Explanation: The given alcohol is a tertiary alcohol and can easily form alkyl halides with HCl and does not require any catalyst. However, primary and secondary alcohols require ZnCl2 as catalyst in the same reaction for it to proceed.
15. Propan-2-ol undergoes acidic dehydration relatively at a lower temperature than propan-1-ol.
a) True
b) False
View Answer
Explanation: Acidic dehydration of alcohols occurs through the formation of a carbocation. Since tertiary alcohol produce the most stable carbocations and primary alcohols produce the least stable carbocation, the dehydration of tertiary and secondary alcohols is relatively easier.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
