This set of Class 12 Chemistry Chapter 10 Multiple Choice Questions & Answers (MCQs) focuses on “Haloalkanes & Haloarenes – Chemical Reactions – 3”.
1. What is the correct order of reactivity of haloalkanes towards β-elimination reactions?
a) 1°>2°>3°
b) 3°>2°>1°
c) 1°>3°>2°
d) 3°>1°>2°
View Answer
Explanation: The haloalkanes which will form more stable (highly substituted) alkenes are the ones that will react the fastest. Since tertiary alkyl halides will form the most stable alkenes, they are the most reactive.
2. How many β-carbon atoms does 2-Bromobutane have?
a) 0
b) 1
c) 2
d) 3
View Answer
Explanation: A beta carbon is the carbon atom that is adjacent to the carbon atom that is attached to the halogen. In 2-Bromobutane, the halogen is at position C-2, so the alpha carbon has one carbon on either side, making it two beta carbons
3. Which of the following is the suitable medium for preparing Grignard reagents?
a) Dry acetone
b) Dry ether
c) Concentrated HCl
d) Alcoholic KOH
View Answer
Explanation: When haloalkanes react with magnesium metal in dry ether, special organo-metallic compounds called Grignard reagents are formed.
4. What is formed when ethyl magnesium bromide reacts with water?
a) Grignard reagent
b) Ethane
c) Ethanol
d) Magnesium hydroxide
View Answer
Explanation: Ethyl magnesium bromide is a Grignard reagent and is highly reactive to any proton giving source because of the polar nature of bonding present in it. It forms hydrocarbons when reacted with water.
5. What will be the product of the following reaction?
2CH3CH2Br + 2Na + dry ether = ________
a) Ethane
b) 1-Bromoethane
c) Butane
d) 1-Bromobutane
View Answer
Explanation: Alkyl halides react with sodium metal in dry ether to form hydrocarbons that have double the number of carbon atoms as that in the alkyl halide. This is called the Wurtz reaction.
6. Compared to haloalkanes, the reactivity of haloarenes towards nucleophilic substitution reactions is _________
a) low
b) high
c) very high
d) equal
View Answer
Explanation: Haloarenes form resonating structures in which the carbon-halogen bond acquires a partial double bond character and its cleavage is difficult. Hence, they are less reactive towards nucleophilic substitution reactions.
7. The C-Cl bond length in chlorobenzene is less than the C-Cl bond length in chloromethane because of the ________
a) resonance effect in haloarenes
b) difference in hybridisation of carbon in C-Cl bond
c) instability of phenyl cation
d) possible repulsion between nucleophile and chlorobenzene
View Answer
Explanation: In chlorobenzene, the C of C-Cl bond is sp2 hybridised, whereas the C of C-Cl bond in chloromethane is sp3 hybridised. Thus, there is more s character in the carbon of the C-Cl bond in chlorobenzene and as a result holds the electron pair of C-Cl bond more tightly.
8. Which of the following compounds is most easily converted to a phenol when heated with aqueous NaOH solution?
a) Chlorobenzene
b) 4-Chloronitrobenzene
c) 2,4-Dinitrochlorobenzene
d) 2,4,6-Trinitrochlorobenzene
View Answer
Explanation: The presence of electron withdrawing groups like NO2 at ortho and para positions with respect to the halogen, increases the reactivity of the haloarene. Chlorobenzene has no such group and converts to phenol at the highest temperature and pressure.
9. 2-Chloronitrobenzene is more reactive than chlorobenzene.
a) True
b) False
View Answer
Explanation: Although NO2 is an electron withdrawing group, it is present at meta position with respect to chlorine in 2-Chloronitrobenzene and has no effect on the reactivity of the haloarene. This is because none of the resonating structures of m-nitrobenzene bear the negative charge on the carbon atom bearing the nitro group and as a result the presence of NO2 at meta position does not stabilize the negative charge.
10. Identify the catalyst in Friedel-Crafts acylation reaction.
a) Anhydrous FeCl3
b) Anhydrous AlCl3
c) NaNO2
d) Alcoholic KOH
View Answer
Explanation: Friedel-Crafts acylation is carried out by treating a haloarene with and acetyl chloride in the presence of anhydrous aluminium chloride as a catalyst.
11. What is the major product formed when chlorobenzene reacts with nitric acid in concentrated sulphuric acid?
a) Nitrobenzene
b) 1-Chloro-2-nitrobenzene
c) 1-Chloro-3-nitrobenzene
d) 1-Chloro-4-nitrobenzene
View Answer
Explanation: This is the nitration of chlorobenzene which is an electrophilic substitution reaction. The major product is that where the nitro group is present at a para position to Cl.
12. Identify ‘X’ from the reaction shown.
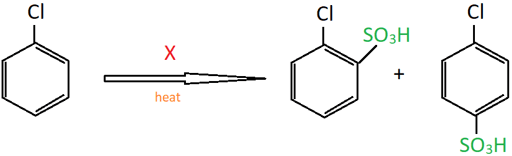
a) Dilute H2SO4
b) Concentrated H2SO4
c) Dilute HNO3
d) Concentrated HNO3
View Answer
Explanation: This is the sulphonation of chlorobenzene which results in the formation of ortho and meta chlorobenzenesulfonic acid.
13. Which is the metal involved in Wurtz-Fittig reaction?
a) Iron
b) Magnesium
c) Aluminium
d) Sodium
View Answer
Explanation: When an aryl halide and alkyl halide and together treated with sodium metal in dry ether, an alkylarene is formed. This is called Wurtz-Fittig reaction.
14. Predict the minor product formed when chlorobenzene reacts with chloromethane in the presence of anhydrous AlCl3?
a) Toluene
b) m-Chlorotoluene
c) o-Chlorotoluene
d) p-Chlorotoluene
View Answer
Explanation: This is the Friedel-Crafts alkylation reaction, where o-Chlorotoluene is the minor product and p-Chlorotoluene is the major product. This is because halogen group is ortho and para directing.
15. Fittig reaction results in the formation of a diphenyl.
a) True
b) False
View Answer
Explanation: Aryl halides give analogous compounds in which two aryl groups are combined when treated with Na in dry ether. This is called Fittig reaction.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Mathematics MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
