This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Diazonium Salts Chemical Reactions – 1”.
1. In reactions of diazonium salts involving the displacement of diazonium group, the nitrogen escapes as _____
a) precipitate
b) liquid
c) gas
d) crystals
View Answer
Explanation: Nitrogen gas is liberated when diazonium salts are treated with suitable reagents so as to displace the N2+X– (X=anion) with an anionic group. This may or may not involve the production of another by-product.
2. Identify the most suitable reagent for converting benzene diazonium chloride to chlorobenzene.
a) CuCl2/HCl
b) Cu2Cl2/HCl
c) CuSO4/HCl
d) CuSO2/H2SO4
View Answer
Explanation: The Cl– nucleophile can be introduced in the benzene ring in the presence of Cu(I) ion which is produced by Cu2Cl2 in hydrochloric acid. This on warming with the diazonium salt will give chlorobenzene.
3. Which of the following cannot be formed from Sandmeyer reaction on benzenediazonium chloride?
a) Chlorobenzene
b) Bromobenzene
c) Iodobenzene
d) Benzonitrile
View Answer
Explanation: Sandmeyer reaction is used for the introduction of chloride, bromide and cyanide ions only in the benzene ring of diazonium salts, by replacing the diazo group. Iodobenzene is produced by another reaction.
4. Identify Y in the following reaction.
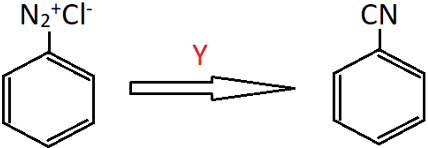
a) Cu2Cl2/KCN
b) CuCN/KCN
c) Cu2Cl2/HCN
d) CuCN/HCN
View Answer
Explanation: When benzenediazonium chloride is treated with copper cyanide in aqueous potassium cyanide solution, benzonitrile is formed. This is also categorized under Sandmeyer reaction.
5. Which of the following can be produced by Gatterman reaction of diazonium salts?
a) Bromobenzene
b) Fluorobenzene
c) Nitrobenzene
d) Cyanobenzene
View Answer
Explanation: Gatterman reaction is a modification of Sandmeyer reaction. It is used for introducing chlorine or bromine in the benzene ring by treating diazonium salt (aqueous) with corresponding halogen acid (HCl or HBr) in the presence of Cu powder.
6. The yield of chlorobenzene from Sandmeyer reaction is better than that from Gatterman reaction.
a) True
b) False
View Answer
Explanation: In Gatterman reaction, there is an additional by product along with chlorobenzene and nitrogen gas, which is a copper salt (with the anion of diazonium salt). The contribution towards formation of this product results in the yield on chlorobenzene to be slightly less.
7. Which of the following compounds has to be used in Gatterman reaction?
a) Chloroform
b) HCl
c) Copper powder
d) Copper chloride
View Answer
Explanation: Gatterman reaction is used for the preparation of either chlorobenzene or bromobenzene by treating a diazonium salt solution with either HCl or HBr respectively, in the presence of copper powder.
8. Benzenediazonium bromide on Gatterman reaction with HCl does not form which of the following?
a) Chlorobenzene
b) Nitrogen gas
c) Copper bromide
d) Copper chloride
View Answer
Explanation: The anion from the diazonium salt (Br–) combines with Cu+ from copper powder (in halogen acid), to form CuBr. The chlorobenzene formed gets the chlorine from HCl in solution.
9. When a diazonium salt is treated with ______ iodobenzene is formed.
a) potassium iodide
b) copper iodide
c) ethyl iodide
d) iodoform
View Answer
Explanation: Iodine is not easily introduced in the benzene ring directly. When an aqueous solution of benzene diazonium salt is warmed with excess potassium iodide, iodobenzene is formed.
10. Which of the following is not produced when C6H5N2+Br– is warmed with KI?
a) C6H5I
b) N2 gas
c) KBr
d) KNO2
View Answer
Explanation: Replacement of diazo group by an iodide ion in benzenediazonium bromide is accompanied by the formation of potassium bromide and liberation of nitrogen gas.
11. Benzenediazonium chloride on treatment with _____ gives a product which on heating forms fluorobenzene.
a) hydrofluoric acid
b) fluoroboric acid
c) potassium fluoride
d) boron trifluoride
View Answer
Explanation: When benzenediazonium chloride is treated with fluoroboric acid, diazonium fluoroborate is formed, which on heating gets decomposed to give fluorobenzene. This is known as Balz-Schiemann reaction.
12. Aryl fluoride can be obtained directly by heating which arenediazonium salt?
a) Benzenediazonium fluoride
b) Benzenediazonium chloride
c) Benzenediazonium hydrogensulphate
d) Benzenediazonium fluoroborate
View Answer
Explanation: Arenediazonium fluoroborate is obtained as a precipitate when arenediazonium chloride is treated with HBF4. This salt on heating decomposes to give aryl fluoride.
13. Which of the following is formed as a by product of decomposition of arenediazonium fluoroborate to give fluorobenzene?
a) BF3
b) BH3
c) H3BO3
d) BN
View Answer
Explanation: Arenediazonium fluoroborate has four fluorine atoms put of which one forms fluorobenzene. The other three fluorine atoms combine with boron from BF4– to form boron trifluoride along with the liberation of nitrogen gas.
14. Alkyl fluorides and iodides cannot be formed from Sandmeyer reaction.
a) True
b) False
View Answer
Explanation: It is difficult to introduced iodide and fluoride ions into the benzene ring and are carried out by separate reactions involving potassium iodide and fluoroboric acid respectively.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Mathematics MCQs
- Check Class 12 - Chemistry Books
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Biology MCQs
