This set of Class 12 Chemistry Chapter 9 Multiple Choice Questions & Answers (MCQs) focuses on “Isomerism in Coordination Compounds – 1”.
1. Two or more compounds that have the same chemical formula, but different arrangement of atoms are called _______
a) isotopes
b) isotones
c) isomers
d) allotropes
View Answer
Explanation: Isotopes are forms of one element due to different number of neutrons in each atom. Isotones are forms of different elements that have the same number of neutrons and allotropes are the different physical forms in which a given element can exist.
2. Which type of isomerism exhibits compounds with same chemical formula and bonds but different spatial arrangement?
a) Optical isomerism
b) Linkage isomerism
c) Structural isomerism
d) Solvate isomerism
View Answer
Explanation: Stereoisomers have the same chemical formula and binds but have different spatial arrangement. Optical isomerism is a type of stereoisomerism.
3. Which of the following is not a subdivision of structural isomerism?
a) Geometrical isomerism
b) Linkage isomerism
c) Coordination isomerism
d) Ionisation isomerism
View Answer
Explanation: Geometrical isomerism is a type of stereoisomerism. Linkage, coordination and ionisation isomers show structural isomerism with different bonds.
4. Geometrical isomerism can be observed in some homoleptic complexes.
a) True
b) False
View Answer
Explanation: Geometrical isomerism takes place only in heteroleptic ligands as there is more than one type of bonding group that can be arranged in different possible manners to give rise to isomers.
5. A coordination complex [MX2L2], has a CN=4 and two unidentate ligands X and L. When the two L ligands are arranged opposite to each other in its geometry, it is called _______ isomer.
a) cis
b) trans
c) fac
d) mer
View Answer
Explanation: The shape of the complex will be square planar as the CN is 4 and isomerism cannot be observed in tetrahedral shapes (also CN=4). When a particular ligand in this type of complex is arranged opposite to each other, it is a trans isomer, else it is called a cis isomer.
6. How many geometrical isomers are possible in [Al(C2o4)3]3-?
a) 0
b) 2
c) 3
d) 4
View Answer
Explanation: The entity shown has a CN=6 as oxalate is a bidentate ligand. The structure of the entity will be same no matter which positions in the geometry each of the oxalate ligands occupy because their relative positions will remain the same in each case.
7. A tetrahedral compound of type [MP2Q2] has two geometrical isomers.
a) True
b) False
View Answer
Explanation: Tetrahedral complexes do not form geometric isomers as the relative positions of all the ligands will always be same because of the geometric properties of a tetrahedron.
8. Identify the trans isomer of [Pt(NH3)2Cl2] from the following.
a) ![The trans isomer of [Pt(NH3)2Cl2] - option a](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q8a.png)
b) ![The trans isomer of [Pt(NH3)2Cl2] - option b](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q8b.png)
c) ![The trans isomer of [Pt(NH3)2Cl2] - option c](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q8c.png)
d) ![The trans isomer of [Pt(NH3)2Cl2] - option d](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q8d.png)
View Answer
Explanation: The compound [Pt(NH3)2Cl2] forms two geometric isomers, one cis and one trans. When the two Cl ligands are arranged adjacent to each other, a cis isomer is formed and when the two Cl ligands are opposite to each other, a trans ligand is formed.
9. How many geometrical isomers are possible in a complex of type [MA2(D)2], where A is unidentate and D is didentate?
a) 0
b) 2
c) 3
d) 4
View Answer
Explanation: In a complex of such type, the two A ligands can be arranged either adjacent to or opposite each other to form cis and trans isomers respectively, making it a total of two possible isomers.
10. The square planar complex [MABCD] is known to form three isomers, two cis and one trans. Shown below are the two cis isomers of the complex. Identify the third trans isomer.
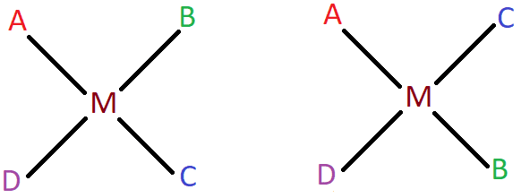
a) ![Third trans isomer of square planar complex [MABCD] - option a](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q10a.png)
b) ![Third trans isomer of square planar complex [MABCD] - option b](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q10b.png)
c) ![Third trans isomer of square planar complex [MABCD] - option c](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q10c.png)
d) ![Third trans isomer of square planar complex [MABCD] - option d](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-isomerism-coordination-compounds-1-q10d.png)
View Answer
Explanation: If you observe the two given cis isomers, it can be seen that they are formed by simply swapping the positions of B and C, but both of them are adjacent to each other in both isomers. So, to obtain the trans isomer, the ligands B and C should lie opposite to each other. The other incorrect options are the same cis isomers but are depicted in different orientations.
11. Which of the following do not show geometrical isomerism? (Assume all ligands are unidentate)
a) Square planar [MXL3]
b) Square planar [MX2L2]
c) Octahedral [MX2L4]
d) Octahedral [MX3L3]
View Answer
Explanation: In square planar complexes, the type [MXL3] does not have any isomers as there is no pair of ligands that can be arranged adjacent to or opposite each other to form cis or trans forms respectively. All possible combinations result in the same spatial arrangement.
12. In the coordination entity [Co(NH3)3(NO2)3], if all three N atoms of the amine ligands occupy adjacent positions at the corners of an octahedral face, the geometrical isomer formed is known as _______ isomer.
a) cis
b) trans
c) fac
d) mer
View Answer
Explanation: Octahedral entities of the type [MA3B3] form two types of isomers based on whether the same ligand groups occupy adjacent positions on the octahedral face (fac) or positions around the meridian of the octahedron (mer).
13. Which of the following compounds has a meridional isomer?
a) [Fe(NO)5Br]+
b) [Al(CO)3(NO2)3]
c) [K(NH)3)4(NO)2]+
d) [Fe(H2O)2(CO)2(NO)2]3+
View Answer
Explanation: Fac-mer isomerism is only exhibited in octahedral coordination entities of the type [MA3B3] depending on the positions of the similar ligands.
14. Optical isomers are also known as __________
a) structural isomers
b) facial isomers
c) meridional isomers
d) enantiomers
View Answer
Explanation: Optical isomers are different forms of the same complex that are mirror images of each other, and which cannot be superimposed. They are chiral complexes also known as enantiomers.
15. The optical isomer that rotates the plane of polarised in the clockwise direction is called ______
a) trans
b) dextro
c) mer
d) laevo
View Answer
Explanation: The optical isomers are of two types based on the direction they rotate the plane of polarised light in a polarimeter. If it rotates it to the right, it is called dextro and if it rotates it to the left, it is called laevo.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Biology MCQs
