This set of Class 12 Chemistry Chapter 11 Multiple Choice Questions & Answers (MCQs) focuses on “Ethers”.
1. Which of the following is the most suitable requirement for the dehydration of ethanol to form an ether?
a) Sulphuric acid at 413K
b) Sulphuric acid at 443K
c) Sodium hydroxide at 413K
d) Sodium hydroxide at 443K
View Answer
Explanation: Alcohols undergo dehydration in the presence of protic acids to form either alkenes or ethers depending upon the reaction temperature conditions. At 413K, ethanol forms an ether with H2SO4.
2. Which of the following is not favourable for the proper dehydration of alcohol to form ether?
a) Good temperature control during reaction
b) Excess of alcohol
c) Presence of protic acids
d) Presence of bulky alkyl groups in the alcohol
View Answer
Explanation: Secondary and tertiary alcohols give alkenes as major products during dehydration, because elimination competes over substitution. Hence, this method is mainly used for primary alcohols.
3. What is the major product formed when ethanol is dehydrated with concentrated H2SO4 at 413K?
a) Ethene
b) Methoxymethane
c) Methoxyethane
d) Ethoxyethane
View Answer
Explanation: Symmetrical ethers are formed when alcohols are dehydrated with H2SO4 at controlled temperatures. This is an SN2 reaction involving the attack of alcohol molecule on a protonated alcohol to give ethers.
4. Sodium methoxide on heating with bromoethane gives __________
a) methoxymethane
b) methoxyethane
c) ethoxyethane
d) diethyl ether
View Answer
Explanation: This is an example of Williamson synthesis of unsymmetrical ether, where CH3ONa is reacted with CH3CH2Br to form CH3CH2OCH3, which is ethyl methyl ether.
5. The reaction between tert-Butyl chloride and sodium ethoxide gives _______
a) tert-Butyl ethyl ether
b) tert-Butyl methyl ether
c) 2-Methylprop-1-ene
d) butene
View Answer
Explanation: The reactant sodium ethoxide is a strong nucleophile as well as a strong base, and hence elimination predominates over SN2 to form alkene as a major product.
6. The boiling point of ethers is ______ the boiling point of alcohols of comparable molecular mass.
a) lower than
b) similar to
c) little higher than
d) much higher than
View Answer
Explanation: The large difference in boiling points of alcohols and ethers is due to the absence of intermolecular bonds in the latter.
7. Ethers are linear molecules with zero dipole moment.
a) True
b) False
View Answer
Explanation: Ethers have angular structures due to the polar nature of C-O bond because of the difference in electronegativity of C and O atoms. Since the two C-O bonds are inclined to each other at about 110°, the dipole do not cancel out, and there is some dipole moment.
8. Ethoxyethane is insoluble in water as compared to butanol.
a) True
b) False
View Answer
Explanation: Ethoxyethane and butanol have the same molecular mass and almost similar solubilities in water, i.e., 7.5g for ethoxyethane and 9g for butanol per 100ml of water. This is because of the fact that even ethers can form hydrogen bonds with water molecules just like the O of alcohols.
9. Which of the following is the least reactive functional group?
a) Alcohols
b) Ethers
c) Aldehydes
d) Ketones
View Answer
Explanation: This is because the (-O-) group in ethers does not contain any active site as compared to for example, hydroxyl group (OH) in alcohols. However, they undergo C-O bond cleavage in drastic conditions.
10. One molecule of dialkyl ether produces how many molecules of alkyl halides with excess of halogen acid?
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: Dialkyl ethers when treated with excess halogen acid first form an alkyl halide and alcohol. The alcohol further reacts with the excess acid to form the same alkyl halide and a water molecule. Thus, two alkyl halide molecules are produced in the end.
11. What product(s) are formed when the shown ether is heated with hydrogen iodide?
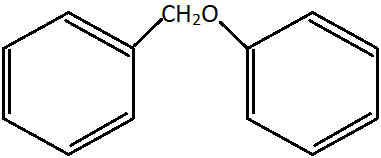
a) (C6H5)CH2I and (C6H5)OH
b) (C6H5)CH2OH and (C6H5)I
c) (C6H5)CH2OH and (C6H5)CH2I
d) (C6H5)OH and (C6H5)I
View Answer
Explanation: The C-O bond of the phenol group is stronger than the C-O bond of the ether and alkyl aryl group. This is due to the partial double bond character because of sp2 hybridised C-O cond. Therefore, the weaker C-O bond is broken and two major products, viz., phenol and iodomethyl benzene, are formed.
12. What is the major product of bromination of anisole in ethanoic acid?
a) o-Dibromobenzene
b) p-Dibromobenzene
c) o-Bromoanisole
d) p-Bromoanisole
View Answer
Explanation: Phenyl alkyl ethers undergo bromination in the benzene ring with bromine in ethanoic acid. This due to the activation of benzene ring by OCH3 group and its ortho, para directing effect. The para isomer is obtained in 90% yield.
13. Identify the major product of Friedel-Crafts acylation of anisole with ethanoyl chloride?
a) 2-Methoxytoluene
b) 4-Methoxytoluene
c) 2-Methoxyacetophenone
d) 4-Methoxyacetophenone
View Answer
Explanation: Anisole undergoes Friedel-Crafts acylation where an acyl group is introduced at ortho and para position by reaction with acyl chloride with anhydrous AlCl3 as catalyst. The para isomer will be the major product.
14. 4-Nitroanisole is obtained as the major product when anisole reacts with _____
a) concentrated H2SO4
b) concentrated HNO3
c) mixture of concentrated H2SO4 and HNO3
d) mixture of dilute H2SO4 and HNO3
View Answer
Explanation: Anisole undergoes nitration in the presence of concentrated acids H2SO4 and HNO3 to yield a mixture of ortho and para nitroanisoles.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
