Here are 1000 Solid State Chemistry MCQ (Chapterwise).
1. What is solid state chemistry?
a) study of the synthesis and characteristics of materials which are in solid phase only
b) study of the synthesis, structure, and characteristics of solid phase materials
c) study of the structure, and characteristics of solid phase materials only
d) None of the mentioned
View Answer
Explanation: Solid-state chemistry, often known as materials chemistry, is the study of the synthesis, structure, and characteristics of solid phase materials, notably non-molecular solids.
2. Which of the following is a classification of solid state chemistry?
a) Crystalline Solid
b) Amorphous Solid
c) Both Crystalline Solid & morphous Solid
d) None of the mentioned
View Answer
Explanation: On the basis of the nature of order present in the arrangement of their constituent particles, solids can be classed as crystalline or amorphous.
3. Which of the following state of matter is Ice?
a) Solid State
b) Gaseous State
c) Liquid State
d) None of the above
View Answer
Explanation: Ice is the solid state of water, which is generally a liquid that freezes to a solid at temperatures below 0 degrees Celsius (32 degrees Fahrenheit).
4. Water Vapor is which state of matter?
a) Solid State
b) Gaseous State
c) Liquid State
d) None of the above
View Answer
Explanation: The gaseous form of water is the third state of water (water vapor). Water molecules travel incredibly quickly and are not bonded together in this condition.
5. What is polymorphism?
a) Existence of substance in more than one liquid modifications
b) Existence of substance in various states
c) Existence of substance in more than one solid modifications
d) Existence of substance in more than one gaseous modifications
View Answer
Explanation: Polymorphism is a condition in crystallography in which a solid chemical compound exists in several crystalline forms, each with somewhat different physical and chemical properties, but identical solutions and vapours.
6. For the preparation of polycrystalline solids (i.e. powder), a mixture of solid starting materials directly react with each other in the solid state. What is the range of the temperature usually required for the solids to react together in Celsius?
a) 25 to 37
b) -25 to 0
c) 2000 to 3000
d) 1000 to 1500
View Answer
Explanation: Solids don’t react usually at room temperature over normal scale timescale, It is required to heat them at much higher temperature often 1000◦C to 1500◦C, in order for a reaction to occur at an appreciable rate.
7. What are the major important factors for the solid state reactions?
a) Mechanical properties of solids
b) Thermodynamic and kinetic factors
c) Environmental factors
d) Size of the particles
View Answer
Explanation: In Solid state reactions thermodynamic factors show whether or not a particular reaction should occur by considering the change in free energy that are involved and kinetic factors determine the rate at which the reaction occurs.
8. The appropriate relation between the surface area of any solid with that of the particle size can be stated as
a) Surface area of any solid is always equal to the particle size
b) Surface area of any solid increases with decrease in particle size
c) Surface area of any solid doesn’t depend at all on the particle size
d) Surface area of any solid increases with increase in particle size
View Answer
Explanation: Surface area is always inversely related with the particle size. In the form of a single crystal, a few grams of solid has a surface area about to equal to the area of a large postage stamp. In the form of a finely divided powder, its surface area is about equal to the area of a hundred meter running track. Hence surface area increases with decrease in particle size.
9. Which one of the following principle has been used in the zone melting method?
a) Impurities concentrate in the liquid phase than in gaseous phase
b) Impurities concentrate in the solid than in liquid phase
c) Impurities concentrate in the gaseous phase than in the solid phase
d) Impurities concentrate in the liquid phase than in the solid phase
View Answer
Explanation: Zone melting method make the use of the principle that impurities usually concentrate in the liquid rather than in the solid phase. Impurities are therefore ‘swept out’ of the crystal by the moving molten zone. The method has been used for the purification and crystal growth of the high melting metals such as tungsten.
10. What is the appropriate relation between the reactions of gases and solids?
a) Gases react faster than the solids
b) Gases do not react at all while solids react faster
c) Both gases and solid reacts at a same rate
d) Gases react slower than the solids
View Answer
Explanation: Gases react much more quickly than do solids because mobilities are increased. In addition, the gaseous phase is often important in normal solid state reactions under isothermal conditions where it may act as a rapid means of transporting matter from one crystal to another.
11. Crystallization of melts is very similar to crystallization of solutions, what is the factor that differentiates the crystallization of melt and solutions?
a) Environmental factors
b) Entropy
c) Size of the particles
d) Temperature
View Answer
Explanation: Melts are high temperature liquids whereas solutions are liquids at low temperatures. Thus on melting together the solid starting materials, complete homogenization occurs and recrystallization takes place on subsequent cooling of the melt.
12. What are the starting materials for crystallization?
a) Liquid, aqueous solution, emulsion
b) Gas, aqueous solution, foam
c) Aqueous solution, melt, glass or gel
d) Solid, gas, melt, solid aerosol
View Answer
Explanation: Aqueous solution, melt, glass or gel, is usually homogenous, single phase and amorphous. This may greatly facilitate formation of crystalline product, since long range diffusion of ions may not be necessary and the product my form at lower temperature.
13. Which one of the following options shows the property of an electrolyte that can be used in the electrolysis process?
a) Solid non conductor
b) Non conducting
c) Solid conductor
d) Conducting
View Answer
Explanation: An electrolyte which is used to achieve electrolysis, is an ion conducting polymer which contains free ions that are able to carry the current in the electrolysis process, if the electrolyte is a solid conductor like solid salts then it will not contain free ions hence it will not be able to carry current, thus electrolysis will not take place.
14. In the chemical vapour deposition the films formed are formed by decomposition of what kind of substances?
a) Gaseous molecules
b) Solid molecules
c) Conducting polymers
d) Liquid molecules
View Answer
Explanation: Chemical vapour deposition the films are formed by deposition of gaseous molecules, the decomposition can be achieved by for example, pyrolysis (i.e. heating), photolysis (i.e. irradiation with IR or UV light) or chemical reaction.
15. Hydrothermal method can be a useful technique for___________
a) Growing single crystals
b) Growing amorphous solid
c) Growing Gaseous particles
d) Growing liquid CO2
View Answer
Explanation: Hydrothermal method can be a useful technique for growing single crystals by arranging for a suitable temperature gradient to be present in the reaction vessel, dissolution of the starting material may occur at the hot end reprecipitation at the cooler end.
16. In the zone melting method _____________ of the charge is melted at any one time. Fill up the correct option for the blank space from the choices given below.
a) Anionic part
b) Solid part
c) Small part
d) Large part
View Answer
Explanation: In zone melting method the thermal profile through the furnace is such that only a small part of the charge is melted at any one time. Initially, that part of the charge in contact with the seed crystal is melted. As the boat is pulled through the furnace, oriented solidification onto the seed occurs and at the same time, more of the charge melts.
17. If the composition of the solid solutions dependence is linear then which of the following law is obeyed?
a) Vegard’s law
b) Mendeleev’s law
c) Stock Barger’s law
d) Bridgeman’s law
View Answer
Explanation: for the solid solutions the composition dependence if linear then Vegard’s law is obeyed, deviation from this law often occur in metallic solid solutions and when they occur can usually be ascribed to a structural feature in the solid solutions.
18. Which of the following is the structure of Graphite?
a) 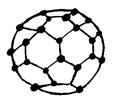
b)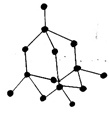
c)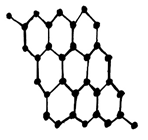
d)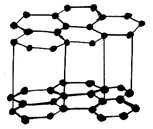
View Answer
Explanation: Graphite has a planar ring structure and it has oblique projection showing the two-layer stacking sequence, it is possible to intercalate, for example, alkali cations, halide anions, ammonia and amines, oxysalts and metal halides between the carbon layers.
19. In which of the following manner the carbon layers are arranged in the graphite structure?
a) ABCABC
b) AAAAAA
c) CBACBA
d) ABABAB
View Answer
Explanation: In graphite structure, the carbon layers are stacked together with a two-layer repeat unit which may be represented as ABABAB. Thus some carbon atoms are directly over the carbon atoms in the layer below whereas others are over the space in the middle of the rings.
20. Which of the following can act as a host crystal during the process of intercalation?
a) Titanium
b) Fluorine
c) Aluminium
d) Graphite
View Answer
Explanation: Graphite ac as a host crystal that is capable of intercalating a wide variety of atoms, ions and molecules. And as such it has been extensively studied. It forms intercalating compounds like graphite fluoride, bronze etc, where it acts as a host crystal.
21. Which of the following is the main component required to carry out the process of electrolysis?
a) Wires
b) DC current
c) Metal vessel
d) AC current
View Answer
Explanation: Electrolysis is a method where DC current i.e. direct electric current is passed through an ionic substance which is either in molten state or may be dissolved in a solvent, thus chemical reactions take place at the electrodes.
22. Which of the following is the main factor because of which DTA cells are designed?
a) Minimum sensitivity
b) Maximum solubility
c) Minimum solubility
d) Maximum sensitivity
View Answer
Explanation: DTA, differential thermal analysis cells are usually designed for maximum sensitivity to thermal changes but this is often at the expense of losing a calorimetric response.
23. A rapid TGA method is used for which of the following process?
a) Decomposition of enzymes exothermally
b) Decomposition of crystals endothermally
c) Decomposition of reactions isothermally
d) Decomposition of polymers exothermally
View Answer
Explanation: An accurate and rapid TGA method is to study the decomposition of reactions isothermally, the TGA furnace is arranged at a pre-set temperature and the sample introduced directly at this temperature. After allowing 2 to 3 minutes for the sample with time can be followed. The process may be repeated at other temperatures and the results analyzed to determine reaction mechanisms, etc.
24. Which of the following statements is true for Kα transition?
a) It is four let
b) It is triplet
c) It is doublet
d) It is singlet
View Answer
Explanation: Kα is a transition from 2p→1s, and it is a doublet because the transition has slightly different energy for the two possible spin states of the 2p electron while makes the transition, relative to the spin of the vacant 1s orbital.
25. Which of the following statement is true for the optical grating?
a) It gives 2 dimensional analogue of the 3 dimensional process
b) It gives 1 dimensional analogue of the 2 dimensional process
c) It gives 1 dimensional analogue of the 3 directional process
d) It gives 3 dimensional analogue of the 1 dimensional process
View Answer
Explanation: Optical grating is a system by which we understand the diffraction of light, so it gives a one dimensional analogue of the three dimensional process that occurs in crystals.
26. Which of the following equation is used for the constructive interference in optical grating?
a) a cotα=nλ
b) a secα=nλ
c) a tanα=nλ
d) a sinα=nλ
View Answer
Explanation: This equation gives the conditions under which constructive interference occurs and relates the spacing of the grating to the light wavelength and the diffraction order, n. Hence, depending on the value of a sinα, one or more diffraction orders, corresponding to n=1,2, etc, may be observed.
27. Which of the following unit cell has all the angles at 90˚ but the sides are of unequal length?
a) Triclinic unit cell
b) Monoclinic unit cell
c) Hex clinic unit cell
d) Orthorhombic unit cell
View Answer
Explanation: The orthorhombic unit cell may be regarded as something like a shoebox in which the angles are all 90˚ but the sides are of unequal length. It usually possesses several mirror planes of symmetry and several twofold axes.
28. Which of the following rays are used in the powder method of crystals?
a) β-rays
b) Monochromatic X-rays
c) Gamma rays
d) α-rays
View Answer
Explanation: A monochromatic beam of X-rays strike a finely powdered sample that ideally has crystals randomly arranged in every possible orientation in the powder method.
29. Which of the following phenomenon takes place when the arrangement of the crystal is not random?
a) Preferred orientation exits
b) Preferred orientation enters
c) The temperature increases
d) The temperature decreases
View Answer
Explanation: If the crystal arrangement is not random, then preferred orientation exists and can introduce errors, sometimes very large, into the measured intensities preferred orientation is a serious problem for the materials that crystallize in a characteristic, very non-spherical shape like clay minerals, etc.
30. Cu Kα radiation of wavelength 1.5418 A˚, is generated by which of the following transition?
a) 2s→4s
b) 2p→1s
c) 1s→2p
d) 3s→1s
View Answer
Explanation: 2p→1s electronic transition generates the Cu Kα radiation of wavelength 1.5418 A˚ and in order to create a vacant 1slevel in the first place, 1s→∞ ionization potential is needed and this energy difference corresponds to a wavelength for copper of1.3804 A˚.
31. Unit cell parameters are determined by which of the following parameters?
a) Double crystal Gamma rays graphs
b) Single crystal Gamma rays graphs
c) Double crystal X-ray photographs
d) Single crystal X-ray photographs
View Answer
Explanation: The unit cell parameters in the crystal are often determined with the aid of single crystal X-ray photographs. The symmetry or unit cell type is first obtained from an inspection of the photographs.
32. Stress is caused on the crystal by which of the following factor?
a) External pressure
b) External strain applied
c) External change in temperature
d) Internal stress
View Answer
Explanation: Stresses on the crystal is caused by the application of an external pressure for example the working hard of the metals in which residual distortions are present in the crystal after treatment.
33. Which one of the following is an additional feature of the focusing cameras?
a) Crystal monochromator
b) Electron stopper
c) X-ray
d) Gamma rays detector
View Answer
Explanation: In the focusing cameras an additional feature is the crystal monochromator which serves two functions, to give highly monochromatic radiation and to produce an intense, convergent X-ray beam.
34. Which of the following shapes are formed by the diffracted radiation in the powder method?
a) Spherical
b) Cubical
c) Circular
d) Conical
View Answer
Explanation: For any set of lattice phases, the diffracted radiation forms the surface of a cone. The only requirement for diffraction is that the planes be at angle Θ to the incident beam, no restriction is placed on the angular orientation of the plane about the axis of the incident beam.
35. Which of the following phenomenon occur when the waves originate from each line source in the optical grating?
a) Interference
b) Magnetic flux
c) Refraction
d) Reflection
View Answer
Explanation: Interference occurs between the waves when they originate from each line source. In certain directions, adjacent beams are in phase with each other and constructive interference occurs to give a resultant diffracted beam in that direction.
36. Which of the following factor is taken care while selecting the container in the process of vacuum evaporation?
a) Withstand high temperature
b) Chemically reactive
c) Conducting
d) Withstand low temperature
View Answer
Explanation: A wide variety of source material for evaporation may be used including metals, alloys, semiconductors, insulators, and inorganic salts. These are placed in the container made of for example tungsten, tantalum or molybdenum, which can withstand very high temperature and chemically unreactive towards the material being evaporated.
37. Which of the following states the second law of electrolysis reaction?
a) Archimedes principle
b) Faraday’s law
c) Henry’s law
d) Einstein equation
View Answer
Explanation: Second law of electrolysis based on Michael Faraday’s second law which states that when the same amount of current is passed through different electrolytes or elements which are connected in series then the mass of the substance liberated or deposited at the electrode is directly proportional to their equivalent weight.
38. Intensities depends on which of the following factors?
a) Emission factor
b) Absorption factor
c) Solubility of the solid
d) Refraction factor
View Answer
Explanation: Intensities depend on several factors, one of its factor is absorption factor, absorption of X-rays by the sample and depend on the form of the sample and geometry of the instrument. Ideally, for single crystal work, crystals should be spherical so as to have the same absorption factor in all directions.
39. Which of the following variables govern the different X-ray techniques?
a) Energy, sample, temperature
b) Heat, mass, proteins
c) Radiation, sample, detector
d) Radiation, diffraction, detector
View Answer
Explanation: In the X-ray diffraction techniques the three variables govern it these are. Radiation-monochromatic or variable of λ. Sample-single crystal, powder or a solid piece, Detector-Radiation counter or photographic film.
Chapterwise Multiple Choice Questions on Solid State Chemistry

- Preparative Methods
- Thermal Analysis
- X-Ray Diffraction
- Descriptive Crystal Chemistry, Point Groups, Space Groups and Crystal Structure
- Some Factors which Influence Crystal Structures
- Crystal Defects and NonStoichiometry
- Solid Solutions
- Phase Transitions
- Interpretation of Phase Diagrams, Ionic Conductivities and Solid Electrolytes
- Electronic Properties and Band Theory
- Other Electrical Properties
- Magnetic Properties, Optical Properties of Luminescence, Lasers
- Glass
- Cement and Concrete, Organic Solid State Chemistry
1. Solid State Chemistry MCQ on Preparative Methods
The section contains Solid State Chemistry multiple choice questions and answers on solid state reaction, melts crystallization, intercalation reactions, electrochemical reduction methods, thin films preparation, single crystals growth, solid survey techniques and its applications, high pressure and hydrothermal methods.
2. Solid State Chemistry Multiple Choice Questions on Thermal Analysis
The section contains Solid State Chemistry questions and answers on thermogravimetric analysis, differential thermal analysis and scanning calorimetry, dta and tga applications.
|
|
|
3. Solid State Chemistry MCQ on X-Ray Diffraction
The section contains Solid State Chemistry MCQs on xrays and its generations, diffraction, definitions, xray diffraction experiment, intensities, powder pattern stress effect, modern x-ray powder techniques and applications, fluorescence radiation, powder patterns structure, crystal symmetry influence and phases mixtures.
|
|
|
4. Solid State Chemistry Multiple Choice Questions on Descriptive Crystal Chemistry, Point Groups, Space Groups and Crystal Structure
The section contains Solid State Chemistry multiple choice questions and answers on point and space groups, crystal structures description, structure types and silicate structures.
|
|
|
5. Solid State Chemistry MCQ on Some Factors which Influence Crystal Structures
The section contains Solid State Chemistry questions and answers on preliminary survey, ionic and polymeric structures, mooser, non bonding electrons effect, bond valence and length.
|
|
|
6. Solid State Chemistry Multiple Choice Questions on Crystal Defects and NonStoichiometry
The section contains Solid State Chemistry MCQs on perfect and imperfect crystals, defects types, point defects, defect clusters, aggregates, interchanged atoms, extended defects, nonstoichiometry defects and solids mechanical properties.
|
|
|
7. Solid State Chemistry MCQ on Solid Solutions
The section contains Solid State Chemistry multiple choice questions and answers on studying solid solutions methods, substitutional and interstitial solids solutions.
|
|
|
8. Solid State Chemistry Multiple Choice Questions on Phase Transitions
The section contains Solid State Chemistry questions and answers on phase transition, buerger’s classification, phase transitions classification, g-t diagrams application, ubbelohde classification, kinetics phase transitions and crystal chemistry.
|
|
|
9. Solid State Chemistry MCQ on Interpretation of Phase Diagrams, Ionic Conductivities and Solid Electrolytes
The section contains Solid State Chemistry MCQs on phase diagrams definitions, one, two and three components condensed systems, typical ionic crystals, solid electrolytes, conductivity measurements, experimental techniques and solid electrolytes application.
|
|
|
10. Solid State Chemistry Multiple Choice Questions on Electronic Properties and Band Theory
The section contains Solid State Chemistry multiple choice questions and answers on metals, insulators and semiconductors, simple band theory refinments, metals and insulators band structures, controlled valency semiconductors, semiconductors application, colour and band structures of inorganic solids.
|
|
|
11. Solid State Chemistry MCQ on Other Electrical Properties
The section contains Solid State Chemistry questions and answers on thermoelectric effects, hall effect, dielectric materials, ferroelectricity, pyroelectricity, piezoelectricity, relationship and application between ferro, pyro and piezoelectricity.
|
|
|
12. Solid State Chemistry Multiple Choice Questions on Magnetic Properties, Optical Properties of Luminescence, Lasers
The section contains Solid State Chemistry MCQs on magnetic properties basics, theory, structures and properties, lasers, luminescence and phosphors.
|
|
|
13. Solid State Chemistry MCQ on Glass
The section contains Solid State Chemistry multiple choice questions and answers on glass formation factors and thermodynamics, kinetics of crystallization, liquid immiscibility, chalcogenide and glass ceramics.
|
|
|
14. Multiple Choice Questions on Cement and Concrete, Organic Solid State Chemistry
The section contains Solid State Chemistry questions and answers on portland cement, topochemical control of solid state organic reactions and electrically conducting organic solids.
|
|
|
Wish you the best in your endeavor to learn and master Solid State Chemistry!
