This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Amines Chemical Reactions – 2”.
1. If p, q and r are the pKb values of methylamine, N-methylamine and N,N-dimethylamine respectively, what is the correct order of p, q and r?
a) p > q > r
b) r > q > p
c) q > p > r
d) r > p > q
View Answer
Explanation: This peculiar order of basic strength is due to a combination of inductive, solvation and stearic effects of the alkyl groups in the amines. In methyl substituted amines, the solvation effect superimposes the inductive effect to make CH3NH2 more basic than (CH3)3N. The 2° compound is the most basic because of its higher inductive effect than primary amine and higher solvation than tertiary amine.
2. What is the correct order of basic strength of the following ethyl substituted amines in aqueous solution? (R=ethyl group)
a) RNH2 > R2NH > R3N
b) R2NH > R3N > RNH2
c) R3N > R2NH > RNH2
d) R2NH > RNH2 > R3N
View Answer
Explanation: There is a subtle interplay of inductive effect, solvation effect and stearic hinderance of alkyl groups to determine the order of basicity of alkylamines in aqueous solutions. For ethyl substituted amines, it is 2°>3°>1°
3. If the pKb value of N,N-diethylethanamine is 3.25, predict the pKb value of ethanamine.
a) 3.00
b) 3.29
c) 4.75
d) 9.38
View Answer
Explanation: In ethyl substituted amines (aqueous), the inductive effect has a bigger role than the solvation effect (because the size of ethyl is larger than methyl group), and as a result the basic strength of N,N-diethylethanamine will be higher than that of ethanamine. Thus, the pKb value of the latter will be very slightly higher than the former.
4. Which of the following aromatic amines has lower pKb value than ammonia?
a) Benzylamine
b) Benzenamine
c) N-Methylbenzenamine
d) N,N-Dimethylbenzenamine
View Answer
Explanation: In aryl amines, the N atom is directly attached to the benzene ring. This makes the lone pair of N to be in conjugation with the benzene ring, thus making it less available for protonation. Whereas, benzylamine is a primary arylalkyl amine and has higher basic strength than ammonia.
5. Which of the following amines will be most reactive when treated with HCl?
a) N-Methylmethanamine
b) N,N-Dimethylmethanamine
c) N-Ethylethanamine
d) N,N-Diethylethanamine
View Answer
Explanation: Between methyl substituted and ethyl substituted aqueous amines, the latter will have relatively higher Kb values due to the ethyl group being larger than methyl group. Of the ethyl substituted amines, the secondary amine will be the most basic because of the combined inductive and solvation effect of alkyl group. Thus, being the most basic, it will react faster with HCl.
6. How many more resonating structures does aniline have than anilinium ion?
a) 2
b) 3
c) 4
d) 5
View Answer
Explanation: Aniline has five resonating structures, out of which three of them have a positive charge on nitrogen. This results in unavailability of electron pair for protonation. When aniline accepts a proton, it forms anilinium ion which has only two resonating structures and is less stable and more basic than aniline.
7. Which of the following groups when present at para position increases the basic strength of aniline?
a) NO2
b) Br
c) NH2
d) COOH
View Answer
Explanation: Electron donating groups at para position release electrons towards the nitrogen atom, stabilizes the anilinium cation and hence increase the basic strength. Since NH2 is an electron donating group, p-phenylenediamine will be more basic than aniline.
8. Identify X if the shown compound has a higher pKb value than aniline.
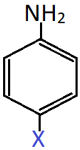
a) OH
b) CH3
c) NH2
d) Cl
View Answer
Explanation: The compound has a higher pKb value than aniline means that it has a lower acidic strength than aniline. This is possible is X is an electron withdrawing group (Cl) that destabilizes the anilinium cation and reduces the basic strength.
9. 4-Aminobenzoic acid has a lower pKa value compared to aniline.
a) True
b) False
View Answer
Explanation: Lower pKa value means more acidic, that is less basic, which means a higher pKb value than aniline. 4-Aminobenzoic acid can be thought of as aniline with COOH group substituted at para position. Since COOH is electron withdrawing in nature, it reduces the basic strength of aniline.
10. Which of the following is the least basic amine?
a) p-Bromoaniline
b) p-Chloroaniline
c) p-Nitroaniline
d) p-Aminobenzonitrile
View Answer
Explanation: All the compounds can be considered as aniline with an electron withdrawing (deactivating) group substituted at the para position to NH2. Since nitro group is the most deactivating group compared to halogen and cyanide, p-nitroaniline is the least basic compound.
11. Which of the following will have the highest pKb value?
a) C6H5NH2
b) p-C6H5(CH3)NH2
c) p-C6H5(OCH3)NH2
d) p-C6H5(NH2)NH2
View Answer
Explanation: CH3, OCH3 and NH2 are all electron donating groups. When these are substituted at para position in aniline, it stabilizes the respective anilinium ion formed and thus increases the basic strength. Therefore, aniline is the weakest base from the given compounds and will have the highest pKb value.
12. Identify the correct order of basic strength of the following substituted anilines?
a) p-Methylaniline > m-Methylaniline > p-Nitroaniline > m-Nitroaniline
b) p-Methylaniline > m-Methylaniline > m-Nitroaniline > p-Nitroaniline
c) p-Nitroaniline > m-Nitroaniline > p-Methylaniline > m-Methylaniline
d) p-Nitroaniline > m-Nitroaniline > m-Methylaniline > p-Methylaniline
View Answer
Explanation: The base weakening effect of electron withdrawing group (NO2) and the base strengthening effect of electron donating group (CH3) is more prominent at para position than at meta position.
13. If ‘a’ is the pKb value of aniline and ‘b’, ‘c’ and ‘d’ are the pKb values of o-, m- and p- isomers of methylaniline respectively, what is the correct order of the values a, b, c and d?
a) a > b > c > d
b) d > c > a > b
c) d > c > b > a
d) b > a > c > d
View Answer
Explanation: Every ortho substituted aniline (electron withdrawing or electron releasing group) is less basic than aniline and consequently its meta and para isomers. This is because of the ortho effect, which is due to the combination of stearic and electronic factors.
14. If the pKb value of p-nitroaniline is 13, predict the pKb value of its ortho isomer?
a) 9.38
b) 11.54
c) 13
d) 14.22
View Answer
Explanation: NO2 is an electron withdrawing group and has a weakening effect on the basicity of anilines. Its presence at para position will be more influencing than at meta, but less weakening than at ortho position. This anomaly is due to a complex ortho effect (stearic and electronic reasons). Hence o-nitroaniline is a weaker base and has a higher pKb value than p-nitroaniline.
15. Phenylmethanamine is more basic than benzenamine.
a) True
b) False
View Answer
Explanation: In arylalkyl amines, the lone pair od electrons on N is not conjugated with the benzene ring and is not delocalized. Hence, the lone pair of electrons on n atom inn arylalkyl amines is more readily available for protonation than that on the N atom of benzenamine.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
