This set of Class 12 Chemistry Chapter 9 Multiple Choice Questions & Answers (MCQs) focuses on “Definitions – Coordination Compounds – 2”.
1. The coordination number is determined by the number of both, sigma and pi bonds formed by the ligand with the central atom/ion.
a) True
b) False
View Answer
Explanation: The CN is found by only considering the number of sigma bonds between the donor atoms and central atom/ion. Pi bonds between ligand and central atom/ion are not counted for this purpose.
2. The coordination polyhedron geometry shown belongs to which of the following complexes?
![The arrangement is tetrahedral & CN=4 if [Co(NH3)6]3+ is octahedral with CN=6](https://www.sanfoundry.com/wp-content/uploads/2020/02/chemistry-questions-answers-definition-some-important-terms-pertaining-coordination-compounds-2-q2.png)
a) [Co(NH3)6]3+
b) [Ni(CO)4]
c) [CoCl2(en)2]2+
d) [Fe(C2O4)3]3-
View Answer
Explanation: The arrangement shown is tetrahedral and CN=4. [Co(NH3)6]3+ is octahedral with CN=6. Both [CoCl2(en)2]2+ and [Fe(C2O4)3]3- have didentate ligands and hence CN=6.
3. What is the correct way of representing nickel in [Ni(CO)4]?
a) Ni(0)
b) Ni(I)
c) Ni(II)
d) Ni(III)
View Answer
Explanation: The oxidation number of a metal is represented as Roman numeral in parenthesis preceded by the name of the entity. Since oxidation number of nickel in the given compound is zero, it is represented as Ni(0).
4. What is the shape of the coordination polyhedron of [PtCl4]2-?
a) Linear
b) Square planar
c) Tetrahedral
d) Octahedral
View Answer
Explanation: The CN of the metal ion is 4 because Cl– is a unidentate ligand. The only possibility for the shape of its coordination polyhedra is square planar or tetrahedral. From crystal field splitting diagram, it is known that [PtCl4]2- is square planar.
5. Complexes in which a metal is attached to only one kind of donor group is called _______
a) Unidentate
b) Chelate
c) Homoleptic
d) Heteroleptic
View Answer
Explanation: Unidentate and chelate are words associated with ligands, whereas coordination complexes can be either homoleptic or heteroleptic depending upon whether only one kind or different kinds of ligands linked to the metal atom/ion respectively.
6. Which of the following compounds consists of a homoleptic complex?
a) [Co(NH3)6]Cl3
b) [Co(NH3)5Cl]Cl2
c) [Co(NH3)4Cl2]Cl
d) [Co(NH3)5(CO3)]Cl
View Answer
Explanation: [Co(NH3)6]Cl3 consists of only ammonia ligands in the complex and is hence homoleptic. The rest of the compounds consists of two kinds of ligates, either NH3 and Cl or NH3 and CO3, and hence they are heteroleptic.
7. The oxidation number of the central metal ion in a coordination entity is the charge it would carry if all the _________ are removed along with the electron pairs that are shared with it.
a) lewis acids
b) anions
c) ligands
d) didentate ligands
View Answer
Explanation: The oxidation number or primary valence of a central atom is its charge if all the ligands and its donor atoms are not present in the coordination entity.
8. The oxidation number of the central metal in a coordination entity is always a positive quantity.
a) True
b) False
View Answer
Explanation: The oxidation number might not always be positive. It can also be 0 or a negative value. For example, the oxidation number of cobalt in K[Co(CO)4] is -1.
9. What is the denticity of the ligand shown in the figure?
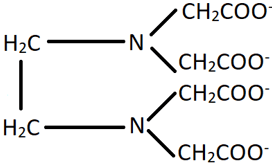
a) 2
b) 4
c) 6
d) 8
View Answer
Explanation: The ion shown in the figure is ethylenediaminetetraacetate which is a hexadentate ligand and can bind through its two N atoms or four O atoms. Hence, its denticity is 6.
10. When a polydentate ligand uses all its donor atoms simultaneously to bond to a single metal ion, it is said to be a _______ ligand.
a) unidentate
b) didentate
c) chelate
d) ambidentate
View Answer
Explanation: Unidentate ligands use only one donor atom at a time. Didentate are polydentate ligands that have two donor atoms. An ambidentate ligand can bind through different donor atoms.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Biology MCQs
