This set of Class 12 Chemistry Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Nomenclature and Structure of Carboxyl Groups”.
1. Butyric acid was first obtained from _______
a) red ants
b) vinegar
c) rancid butter
d) goats
View Answer
Explanation: The common names of some carboxylic acids gives an idea of where it was first obtained from in nature. Butyric acid comes from the Latin word butyrum meaning butter.
2. What is the common name of the simplest dicarboxylic acid?
a) Oxalic acid
b) Malonic acid
c) Acetic acid
d) Propionic acid
View Answer
Explanation: The simplest dicarboxylic acid is ethanedioc acid, which has two carbon atoms both of which are a part of the carboxyl groups. Its formula is (HOOC-COOH).
3. Which of the following acids have a double bond in their structure?
a) Isobutyric acid
b) Succinic acid
c) Acrylic acid
d) Glutaric acid
View Answer
Explanation: The IUPAC name of acrylic acid is prop-2-enoic acid, which indicates the presence of a double bond at the second carbon, the one next to the carboxyl carbon. Its formula is (CH2=CHCOOH).
4. What is the correct IUPAC name of the compound CH3CH=CHCH=CHCOOH?
a) Hexenedioc acid
b) Hexa-2,4-dienoic acid
c) Penta-1,3-dienioc acid
d) Pentenedioc acid
View Answer
Explanation: The compound consists of six carbon atoms including the carboxyl carbon thus, it is a derivative of hexanoic acid. The only substituents are two double bonds at the second and fourth carbon considering that the carboxyl carbon is the number one.
5. Which of the following is not a dicarboxylic acid?
a) Malonic acid
b) Glutaric acid
c) Adipic acid
d) Carballylic acid
View Answer
Explanation: Malonic acid, glutaric acid and adipic acid are dicarboxylic acids with 3, 5 and 6 carbon atoms in their structures respectively. Carballylic acid, also known as tricarballylic acid is a tricarboxylic acid having 3 carbon atoms excluding the three carboxyl carbons in its structure. Its IUPAC name is Propane-1,2,3-tricarboxylic acid.
6. Which of the following has two carboxyl groups in its structure?
a) Succinic acid
b) Crotonic acid
c) Cinnamic acid
d) Mandelic acid
View Answer
Explanation: Succinic acid is a dicarboxylic acid with 4 carbon atoms in its structure. Its IUPAC name is butanedioic acid. Crotonic acid is but-2-enoic acid, whereas cinnamic acid and mandelic acid consists only a single carboxyl group along with a phenyl group.
7. What is the IUPAC name of terephthalic acid?
a) 2-Phenylethanoic acid
b) Benzene-1,2-dicarboxylic acid
c) Benzene-1,3-dicarboxylic acid
d) Benzene-1,4-dicarboxylic acid
View Answer
Explanation: Terephthalic acid is an aromatic dicarboxylic acid with the two carboxyl groups on the opposite ends (para position) of a benzene ring. 2-Phenylethanoic acid consists of only a single carboxyl group.
8. What is the IUPAC name of (C6H5)CH2CH2COOH?
a) 2-Phenylethanoic acid
b) 2-Phenylpropanoic acid
c) 3-Phenylethanoic acid
d) 3-Phenylpropanoic acid
View Answer
Explanation: The compound has the group (-CH2CH2COOH) directly attached to a benzene ring. The preference is given to the carboxyl group and the compound is named as a carboxylic acid derivative. There are total three carbons making it propanoic acid with the benzene ring as substituent on the last carbon (C-3) as a phenyl group.
9. What is the IUPAC name of (CH3)3CCH2COOH?
a) Hexanoic acid
b) 2,2,2-Trimethylpropanoic acid
c) 3,3-Dimethylbutanoic acid
d) 4-Methylpentanoic acid
View Answer
Explanation: The longest parent carbon chain consists of 4 carbon atoms including the carboxyl carbon and one methyl group attached to the tertiary carbon. The other two methyl groups act as substituents on the carbon, which is now the third carbon.
10. What is the correct IUPAC name of the shown compound?
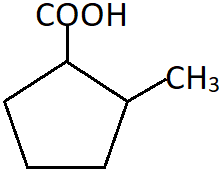
a) 2-Carboxylmethylcyclopentane
b) 2-Methylcyclopentanecarboxylic acid
c) 2-Methylcyclopentanoic acid
d) (2-Methylcyclopentyl)carboxylic acid
View Answer
Explanation: The compound is a cyclic carboxylic acid with five carbon atoms in the cyclic ring and one carboxyl group. The methyl group is present as a substituent at the adjacent position on the ring. Hence, it is named as a derivative of cyclopentanecarboxylic acid.
11. Identify the correct IUPAC name of the following compound?
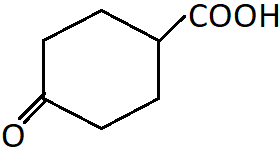
a) 4-Oxocyclohexan-1-carboxylic acid
b) 4-Carboxylcyclohexan-1-one
c) 4-Oxocyclohexylmethanoic acid
d) 4-Oxocyclohexylethanoic acid
View Answer
Explanation: This is a keto-substituted cyclic carboxylic acid and is named considering the CO group as a substituent at the fourth position given that the carbon attached to the carboxyl carbon is C-1.
12. Which of the following is an incorrect name for the following compound?
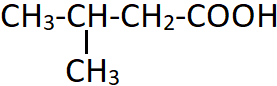
a) α-Methylpropionic acid
b) β-Methylbutyric acid
c) Isovaleric acid
d) 3-Methylbutanoic acid
View Answer
Explanation: The longest parent chain of carbon atoms (including the first carboxyl carbon) is four. The methyl group is substituted at the third carbon, also known as the beta carbon. Isovaleric acid is also a common name for this structure.
13. The carboxyl carbon is more electrophilic than the carbonyl carbon.
a) True
b) False
View Answer
Explanation: The carboxyl group is capable of forming three resonance structures in comparison to the two formed by carbonyl group. This makes the carboxyl carbon less electrophilic than the carbonyl carbon.
14. Salicylic acid consists of an aldehyde group on the benzene ring other than the primary carboxylic group.
a) True
b) False
View Answer
Explanation: Salicylic acid consists of a hydroxy group on the benzene ring at ortho, meta or para position with respect to the carboxyl group. It is named as hydroxybenzoic acid in IUPAC system.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Physics MCQs
