This set of Class 12 Chemistry Chapter 10 Multiple Choice Questions & Answers (MCQs) focuses on “Haloalkanes & Haloarenes – Chemical Reactions – 1”.
1. The reaction that takes place when an electron rich species stronger than the halide approaches the partially positive carbon atom of a haloalkane and forms a new bond with the carbon atom and in the process displacing the halogen is called a ________ reaction.
a) displacement
b) electrophilic substitution
c) nucleophilic substitution
d) elimination
View Answer
Explanation: Nucleophiles are electron rich species and when a stronger nucleophile replaces an already existing one is called a nucleophilic substitution reaction.
2. What will be the class of the nucleophilic substitution product when sodium hydroxide reacts with chloroethane?
a) Alkane
b) Alcohol
c) Amine
d) Ether
View Answer
Explanation: The OH– nucleophile substitutes the Cl– ion in chloroethane to form ethanol, which is an alcohol.
3. Identify the nucleophile in the substitution reaction between water and bromopropane.
a) Br–
b) H+
c) OH–
d) H2O
View Answer
Explanation: The water molecule itself is the nucleophile which has two pairs on electrons on the O atom and attacks the Br atom in the haloalkane.
4. What is the class of the substitution product of the reaction between LiAlH4 and an alkyl halide?
a) Haloalkane
b) Hydrocarbon
c) Nitroalkane
d) Alkyl nitrite
View Answer
Explanation: The nucleophile in LiAlH4 is the H atom which attacks the halogen in the alkyl halide and substitutes it to form the basic hydrocarbon.
5. Which of the followings reagents forms an isonitrile when reacted with an alkyl halide?
a) KCN
b) AgCN
c) KNO2
d) AgNO2
View Answer
Explanation: AgCN has a lone pair of electrons on the N atom and attacks from the N side to forms a compound of the type RNC, where R is the alkyl group.
6. Which of the following is not an ambident nucleophile?
a) Hydroxide
b) Thiocyanate
c) Cyanide
d) Nitrite
View Answer
Explanation: Ambident nucleophiles are those that have two nucleophilic centres and can link through any of their two atoms to result in the formation of different compounds.
7. Identify the nucleophile that gives a primary amine and hydrogen bromide on reaction with bromoethane.
a) NH2
b) NH3
c) H2O
d) H
View Answer
Explanation: Ammonia reacts with alkyl halides to undergo nucleophilic substitution and form amine and a mineral acid.
8. Which of the following statements in incorrect regarding SN2 mechanisms?
a) The rate of the reaction depends on the concentration of both reactants
b) The complete mechanism takes place in a single step
c) The transition state is stable
d) There is inversion of configuration
View Answer
Explanation: In the transition state of SN2 mechanisms, the carbon atoms is simultaneously bonded to the incoming nucleophile and the outgoing group and is hence bonded to five atoms at the same time. Such a geometry is unstable and cannot be isolated.
9. The reaction between chloroethane and potassium hydroxide is a _______ order reaction.
a) zero
b) first
c) second
d) third
View Answer
Explanation: This nucleophilic reaction follows SN2 mechanism and its rate depends on the concentration of both chloroethane and hydroxide ions. Hence, it is a bimolecular reaction.
10. A haloalkane is known to have an SN2 reaction rate 30 times faster than that of ethyl bromide. Identify the haloalkane.
a) Methyl bromide
b) Isopropyl bromide
c) tert-Butyl bromide
d) neo-Pentyl bromide
View Answer
Explanation: The presence of bulky groups around the C atom has an inhibiting effect on the rate of SN2 reaction. Since methyl bromide has only three H atoms around the C, it will have a higher rate than ethyl bromide.
11. Which of the following will have the highest reactivity towards SN2 reaction, where R=methyl group?
a) R-F
b) R-Cl
c) R-Br
d) R-I
View Answer
Explanation: Iodine is a better leaving groups among the halogens as it has a better ability to accommodate the negative charge.
12. Which of the following statements is correct regarding unimolecular nucleophilic substitution reactions?
a) It takes place in three steps.
b) The rate of the reaction depends on the concentration of all the reactants.
c) The first step is the slowest and determines the rate of reaction.
d) None of the steps are reversible.
View Answer
Explanation: SN1 reactions follow first order kinetics and take place in two steps, of which the first one is the slowest, rate determining and reversible step.
13. The rate of the following reaction depends on the concentration of which reactant(s)/?
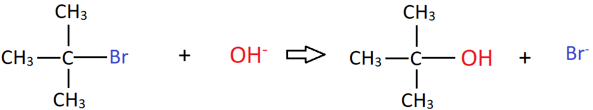
a) tert-Butyl bromide
b) Hydroxide ion
c) Both tert-Butyl bromide and hydroxide ion
d) None of the reactants
View Answer
Explanation: This is an example of a SN1 reaction, where the rate depends on the concentration of only the alkyl halide, because that will determine the concentration of carbocation formed in the first step.
14. Tertiary alkyl halides show higher reactivity towards bimolecular nucleophilic substitution than secondary alkyl halides.
a) True
b) False
View Answer
Explanation: Tertiary alkyl halides have more alkyl groups present around the carbon linked to the halogen, which causes steric hinderance of the nucleophile and hence the reactivity towards SN2 reactions reduces significantly.
15. Iodomethane reacts faster than bromomethane in SN2 reaction with OH–?
a) True
b) False
View Answer
Explanation: Iodine is a better leaving group because of its large size and will be released at a faster rate in the presence of a nucleophile.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Physics MCQs
