This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Structure of Amines”.
1. Which of the following is not an amine?
a) NH3
b) CH3NH2
c) C6H5NH2
d) CH3NHCH3
View Answer
Explanation: Amines are clearly defined as ‘derivatives’ of ammonia obtained by replacement of one or more H atoms by an alkyl/aryl group. Therefore, NH3 itself is not an amine, but the base compound for all amines.
2. Amines are derivatives of ______
a) NCl3
b) NH3
c) N2
d) CH3NH2
View Answer
Explanation: There are three hydrogen atoms in ammonia. When one or more of these is substitutes by an alkyl/aryl group, the resultant compound is an amine. Hence, they are derivatives of NH3.
3. How many lone pairs of electrons does the nitrogen atom of amines have?
a) 0
b) 1
c) 2
d) 3
View Answer
Explanation: The nitrogen of NH3 forms sp3 hybridised orbitals and also a valency of three (as it is attached to three hydrogen atoms). This results is one the orbitals having a lone pair of electrons, resulting in one unshared electron pair on nitrogen.
4. What is the geometry of ammonia molecule?
a) Trigonal planar
b) Square planar
c) Linear
d) Pyramidal
View Answer
Explanation: The nitrogen atom of ammonia undergoes hybridisation to form 4 sp3 orbitals. Three of these overlap with s orbital of H forming three N-H bonds. This results in a pyramidal geometry with N atom at the apex and three H atoms at the corners of a triangle base.
5. What is the expected geometry of CH3-NH-CH3?
a) Square planar
b) Trigonal planar
c) Trigonal pyramidal
d) Trigonal bipyramidal
View Answer
Explanation: CH3-NH-CH3 is an amine in which two hydrogen atoms are replaced by methyl groups. The hybridisation of N atom of amines is same as that of ammonia, and is also expected to have a pyramidal geometry, with N at the apex and the groups CH3, H and CH3 occupying the corners of a trigonal base.
6. What is the bond angle in ammonia molecule?
a) 106.5°
b) 107°
c) 108°
d) 109.5°
View Answer
Explanation: The bond angle for a standard tetrahedral geometry is 109.5°. But the ammonia molecule contains a lone electron pair on nitrogen. As it is closer to the N atom than the other orbitals, it creates a repulsive effect and pushes the Hydrogen atoms close to each other, thus reducing the bond angle to 107°.
7. What is the C-N-C bond angle in the following compound?
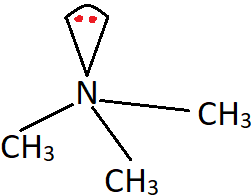
a) 107°
b) 108°
c) 109°
d) 109.5°
View Answer
Explanation: The shown compound is an amine with three methyl group. This induces a steric effect between the three alkyl groups, and results in a slight increase in bond angle from 107° (bond angle in ammonia).
8. An amine with two or more alkyl groups does not have a lone pair of electron on nitrogen atom.
a) True
b) False
View Answer
Explanation: Amines are derivatives of ammonia. Nitrogen of NH3 is sp3 hybridised with one orbital having unshared pairs of electrons as it bonds to only three H atoms. Similarly, amines also have an electron lone pair on nitrogen.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Biology MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Mathematics MCQs
