This set of Class 12 Chemistry Chapter 11 Multiple Choice Questions & Answers (MCQs) focuses on “Structures of Functional Groups”.
1. How many lone pairs of electrons does O atom have in methanol?
a) 0
b) 1
c) 2
d) 4
View Answer
Explanation: The oxygen atom in methanol is sp3 hybridised. One of the sp3 orbitals overlaps with 1s orbital of hydrogen (of OH group) and one sp3 orbital overlaps with sp3 orbital of C atom. The remaining two sp3 orbitals contain one lone pair of electrons each.
2. What is the value of C-O-H bond angle in phenol?
a) 108°
b) 108.9°
c) 109°
d) 109.8°
View Answer
Explanation: The bond angle C-O-H in phenols is 109° which is slightly more than the C-O-H bond angle in methanol (108.9°) and slightly less than the tetrahedral bond angle (109.5°).
3. If A and B are the C-O bond lengths in methanol and phenol respectively, what is the relationship between A and B?
a) A>B
b) A<B
c) A=B
d) A≥B
View Answer
Explanation: The C-O bond length in methanol is 142pm, whereas the C-O bond length in phenol is 136pm, which is slightly less than that in methanol.
4. Which of the following is not a correct reason for the C-O bond length in phenol to be less than that in methanol?
a) Partial double bond character of C-O bond
b) Conjugation of lone pair of electrons with the aromatic ring
c) sp2 hybridised sate of carbon of C-O bond
d) Repulsion between the electron lone pairs of oxygen
View Answer
Explanation: The two lone pairs of electrons of oxygen are present in OH groups of both phenol and methanol and has an effect on the C-O-H bond angle and not the C-O bond length.
5. If the dipole moment of methanol is 1.71D, predict the dipole moment of phenol.
a) 1.54D
b) 1.71D
c) 1.89D
d) 1.96D
View Answer
Explanation: Phenol has a smaller dipole moment than methanol because the C-O bond in phenols is polar due to the electron withdrawing effect of the aromatic ring.
6. The C-O-C bond angle in ethers is _______
a) lesser than 109.5°
b) 109.5°
c) greater than 109.5°
d) 180°
View Answer
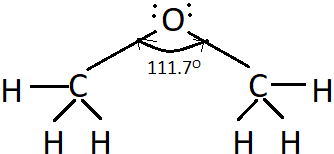
Explanation: Due to the repulsive interaction of the bulky alkyl/aryl groups in ethers, the bond angle is slightly more than the tetrahedral angle, i.e., 109.5°. For example, the C-O-C bond angle in methoxymethane is 111.7°.
7. In methanol, the O-H bond length is smaller than the C-O bond length.
a) True
b) False
View Answer
Explanation: Since hydrogen atom is smaller than the carbon atom and has only 1s orbital, the O-H bond (96pm) is much smaller than the C-O bond (142pm).
8. If the C-O bond length in methanol is 142pm, what will be the C-O bond length in the given compound?
a) 96pm
b) 121pm
c) 141pm
d) 202 pm
View Answer
Explanation: The shown compound in methoxymethane which is an ether. The C-O bond length in ethers is almost the same as that in alcohols.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
