This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Diazonium Salts Chemical Reactions – 2”.
1. The complete reaction for the conversion of aniline to benzene, involving the reduction of diazonium salt, is known as _______
a) diazotisation
b) deamination
c) sandmeyer reaction
d) gatterman reaction
View Answer
Explanation: The complete process of diazotisation of aniline followed by the reduction of diazonium salt or replacement of the diazo group by hydrogen is called deamination.
2. When benzenediazonium chloride is treated with hypophosphorous acid, the product obtained is __________
a) phenol
b) chlorobenzene
c) benzene
d) aniline
View Answer
Explanation: When benzenediazonium salts are treated with mild reducing agents like hypophosphorous acid, the complete diazo and anionic group is replaced by hydrogen atom to form benzene.
3. A diazonium salt, on treatment with which of the following gives benzene?
a) LiAlH4
b) pyridine
c) CH3CH2OH
d) HBF4
View Answer
Explanation: Ethanol is a mild reducing agent which on treatment with a benzenediazonium salt, oxidises to the corresponding aldehyde, and in the process reducing the diazonium salt to give benzene.
4. Identify ‘Y’ in the following reaction.
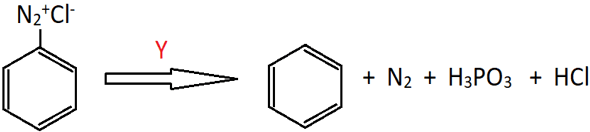
a) PCl3
b) H3PO4
c) H3PO3
d) H3PO2
View Answer
Explanation: In the reaction, benzenediazonium chloride is reduced to benzene. This is possible in the presence of a reducing agent. Since, one of the by products is H3PO3, it must be formed from the oxidation of the reducing agent, and hence must either have less O atoms or more H atoms than in H3PO3. Therefore, it is H3PO2.
5. A diazonium salt ‘X’ forms sulphuric acid as one the by product when is reacts with phosphinic acid to give benzene. Identify ‘X’.
a) Benzenediazonium chloride
b) Benzenediazonium bromide
c) Benzenediazonium hydrogensulphate
d) Benzenediazonium fluoroborate
View Answer
Explanation: The H+ ions from the aqueous solution of acid combine with the negative HSO4– ions from the benzenediazonium hydrogensulphate to form H2SO4 (sulphuric acid) as the by product.
6. Nitrogen gas is evolved as a product of the reaction between benzenediazonium salt and ethanol.
a) True
b) False
View Answer
Explanation: The nitrogen from the diazo group of the diazonium salt is liberated as free nitrogen molecule (N2) is the form of a gas, as a by-product during the reaction.
7. Which of the following pathways will result in the formation of benzene?
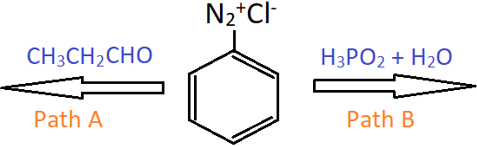
a) Only A
b) Only B
c) Both A and
d) Neither A nor B
View Answer
Explanation: Hypophosphorous acid (H3PO2) is a mild reducing agent, and reacts with benzenediazonium chloride to form benzene. Propionaldehyde does not reduce diazonium salts to benzene.
8. Which of the following is not obtained as a product of the reaction between benzenediazonium bromide and ethanol?
a) Benzene
b) Nitrogen gas
c) Acetic acid
d) Hydrogen bromide
View Answer
Explanation: Ethanol is a mild reducing agent that reduces benzenediazonium bromide to benzene, and itself gets oxidized to an aldehyde, ethanal (CH3CHO).
9. Warming an aqueous solution of benzenediazonium chloride gives phenol.
a) True
b) False
View Answer
Explanation: If the temperature of benzenediazonium salt solutions is allowed to rise upto 283K, the diazonium group is replaced by OH group, and therefore the salt get hydrolysed to phenol.
10. Which of the following is the most suitable reagent for converting benzenediazonium fluoroborate to nitrobenzene?
a) NaNO2/HCl
b) NaNO2/Cu powder
c) HBF4/HNO2
d) HNO3/HCl
View Answer
Explanation: Benzenediazonium fluoroborate on treatment with an aqueous solution of sodium nitrite in the presence of copper (from copper powder), gives nitrobenzene.
11. What is the most suitable temperature for the conversion of benzenediazonium fluoroborate to nitrobenzene, with sodium nitrite and copper?
a) 0°C
b) 10°C
c) 22°C
d) 40°C
View Answer
Explanation: When diazonium fluoroborate is heated with aqueous NaNO2 in the presence of copper metal, the diazo group is replaced by NO2 group, to give nitrobenzene.
12. Which of the following is a by product of the reaction between fluoroborate diazonium salt and sodium nitrite in the presence of copper?
a) HBF4
b) NaBF4
c) Na3N
d) NaF
View Answer
Explanation: The diazonium salt dissociates in solution giving a free fluoroborate anion (BF4–). This combines with the Na+ ions in solution to form NaBF4.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Mathematics MCQs
- Check Class 12 - Chemistry Books
