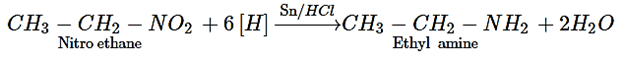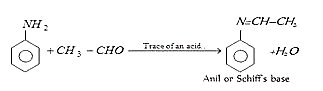Here are 1000 MCQs on Organic Chemistry (Chapterwise).
1. Which of the following is the simplest member of organic compounds?
a) Formic acid
b) Formaldehyde
c) Methane
d) Methanol
View Answer
Explanation: Methane is the simplest member of the alkane family and indeed the simplest of organic compounds, as all other compounds are derived by altering this compound.
2. Which of the following is the known name for the reaction given below?
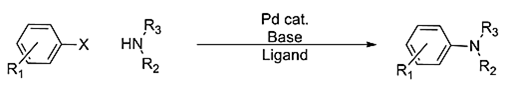
(where, X=Cl, Br, I, OTf; R2=Alkyl, aryl, H; R3=alkyl, aryl)
a) Ullmann reaction
b) Gabriel phthalimide synthesis
c) Buchwald-Hartwig Reaction
d) Chan-Lam coupling
View Answer
Explanation: The Buchwald–Hartwig amination is a chemical reaction used in organic chemistry for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed cross-coupling of amines with aryl halides.
3. Which of the following is yielded when Ethylene glycol is treated with phosphorus tri-iodide?
a) ethylene di-iodide
b) ethylene
c) ethane
d) ethyl iodide
View Answer
Explanation: Phosphorus triiodide (PI3) is an unstable red solid which reacts violently with water. It is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides.
4. Hydrocarbons are organic compounds with element __________
a) Both hydrogen and carbon
b) Carbon
c) Hydrogen
d) Oxygen
View Answer
Explanation: These organic compounds are made up of only carbon and hydrogen and hence the name hydrocarbons.
5. Which of the following bond is made up of a large number of organic compounds?
a) Metallic bond
b) Dipolar bond
c) Ionic bond
d) Covalent bond
View Answer
Explanation: This is because they have the tendency to transfer electrons.
6. Which of the following is a classification of Organic compounds?
a) alicyclic compounds and acyclic compounds
b) Cyclic compounds and alicyclic compounds
c) Open chain compounds and acyclic compounds
d) Open chain compounds and linear chain compounds
View Answer
Explanation: Organic compounds are broadly classified into open chain and closed chain compounds.
7. Which of the following is not a class of organic compounds?
a) Amides
b) Electro compounds
c) Nitro compound
d) Carbonyl compound
View Answer
Explanation: Classes of organic compounds are those which involves organic compounds such as carbon, hydrogen and oxygen. Hence, electro compounds is not a class of organic compounds.
8. Organic compounds can be classified even based upon the function groups. Which of the following is not a functional group?
a) Isocyano
b) Carbonyl
c) Isocyanide
d) Carboxyl
View Answer
Explanation: Isocyanide is a compound and it is not a functional group.
9. Which of the following does not come under the organic addition reaction?
a) Halogenation
b) Hydrohalogenation
c) Hydration
d) Dehydration
View Answer
Explanation: Dehydration comes under elimination reaction and hence it does not come under addition reaction.
10. Which of the following organic compound with molecular formula C3H C12 exhibits only one signal in the IH NMR spectrum?
a) 1, 2-dichloropropane
b) 1, 1-dichloropropane
c) 2, 2-dichloropropane
d) 1, 3-dichloropropane
View Answer
Explanation: Only (a) compound has all chemically equivalent hydrogen. So, give only one peak.
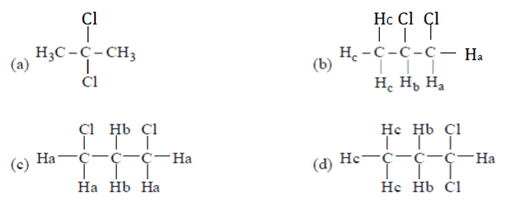
11. Which of the following is an organic compound having the molecular formulae C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR?
a) 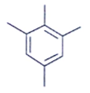
b) 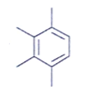
c) 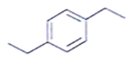
d) 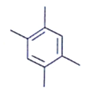
View Answer
Explanation: In compound a;
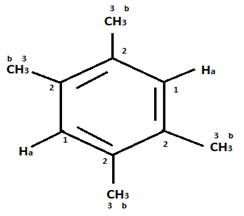
Here only 2 types of Hydrogen are present (two Ha & twelve Hb). So, 2 singlets only. There is no need to check 13C NMR. Still, if you check three types carbon are present (two C1, four C2 and four C2 carbon). Hence, there peak for 13C NMR.
12. An organic compound (MF; C8H10O) exhibited the following 1H NMR special data: 62.5 (3H, s), 3.8 (314, s), 6.8 (2H, d, J 8 Hz), 7.2 (2H, d, J 8 Hz) ppm. Which of the following is that compound among the choices?
a) 4-methylbenzyl alcohol
b) 4-methyl anisole
c) 4-ethylphenol
d) 2-ethylphenol
View Answer
Explanation: C8H10O
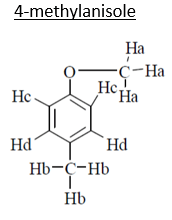
3Ha –> 3.8, singlet (deshielded because of –I of oxygen)
3Hb –> 2.5, singlet (No such –I)
2Hc –> 7.2, doublet (deshielded because of anisotropy effect of benzene as well as because of –I of oxygen)
2Hd –> 6.8, doublet (deshielded because of anisotropy of benzene).
13. Which of the following complex is in which organic ligand is having only bond with metal?
a) (η5-C5H5)2Fe
b) (η5-C6H6)2Ru
c) W(CH3)6
d) K[PtCl3(C2H4)]
View Answer
Explanation: CH3 is the only σ-donor ligand while C2H4, C5H5, C6H6 are π-acceptor ligands.
14. Which of the following statement is not true about ethers?
a) Lower ethers also act as anaesthetics
b) Simple ethers (such as diethyl ether) are tasteless
c) The lower ethers are highly volatile and flammable
d) Ethers are not organic solvents
View Answer
Explanation: Because like dissolve like, Diethyl ether is very non-polar compared to water so it will dissolve the non-polar substances and precipitate the ionic compounds. Since like dissolves like, your barely-polar organic ether is often happy to dissolve your non-polar organic molecules.
15. An organic compound A reacts with sodium metal and forms B. On heating with conc. H2SO4, A gives diethyl ether. What are A and B?
a) C4H9OH and C4H9ONa
b) CH3OH and CH3ONa
c) C2H5OH and C2H5ONa
d) C3H7OH and CH3ONa
View Answer
Explanation: When C2H5OH reacts with sodium metal and forms C2H5ONa and on heating with H2SO4 giver diethyl ether.
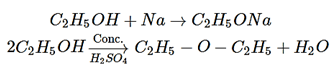
16. An organic compound Q exhibited the following spectral data obtained by mass spectroscopy.
IR: 1760 cm–1
1HNMR: chemical reference (ppm): 7.2 (IH, d, 16.0 Hz), 5.1 (IH, m), 2.1 (3H, s), 1.8 (3H, d, J = 7.0 Hz)
13CNMR chemical reference (ppm): 170 (carbonyl carbon). Which of the following is compound Q?
a)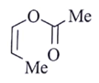
b)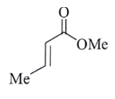
c)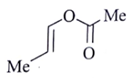
d)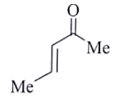
View Answer
Explanation:
IR: 1760 cm–1
Some options are not possible because of IR data.
1760 cm–1 –> Represent either ester or anhydride
ester –>with cross conjugation or anhydride
chemical reference: 7.2 (1H), d, J = 16.0 Hz
This ‘J’ shows it is a trans alkene.
17. Which of the following is the major organic product obtained from the following reaction?
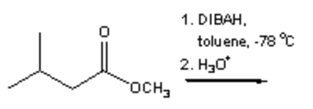
a) 
b) 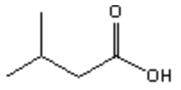
c) 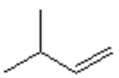
d) 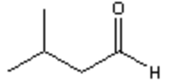
View Answer
Explanation: Di-isobutyl aluminium hydride (DIBAH) is a selective reducing agent. It does not reduce esters to 1° alcohols (lithium aluminium hydride can be used to reduce esters to 1° alcohols).
18. Which of the following is true about transesterification?
a) exchanging the organic alkyl group of alcohol with the organic group alkyl of an ester
b) exchanging the organic alkyl group of an ester with the organic group alkyl of an ether
c) exchanging the organic alkyl group of an ester with the organic group alkyl of an alcohol
d) exchanging the organic alkyl group of an ester with the organic group alkyl of an alkane
View Answer
Explanation: Transesterification is the process of exchanging the organic group R″ of an ester with the organic group R′ of an alcohol.
19. Which of the following is the general formula of Diazonium salt?
a) RN2+HSO–2
b) RN2+X–
c) RN+
d) RXI
View Answer
Explanation: Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R−N2+X– where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halogen.
20. Which of the following is the major organic product obtained from the following reaction?
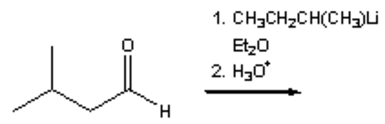
a) 3,5-dimethyl-3-heptanol
b) 3,5-dimethyl-4-heptanol
c) 4,7-dimethyl-4-heptanol
d) 2,4-dimethyl-4-heptanol
View Answer
Explanation: It is also important that products are identified accurately using IUPAC nomenclature: 4,7-Dimethyl-4-heptanol is not an IUPAC name.
21. Which of the following will be the product ‘a’ in the given reation?
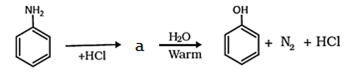
a) Chlorobenzene
b) Benzene
c) Benzene diazonium chloride
d) Enolate form
View Answer
Explanation: When an aromatic primary amine is treated with nitrous (NaNO2 + HCl) acid at 273 – 278 K, diazonium salts are obtained. Upon warming with water, these diazonium salts finally hydrolyze to phenols.
22. Which of the following is the major organic product obtained from the following reaction?
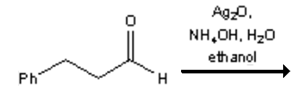
a) 
b) 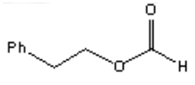
c) ![]()
d)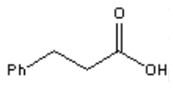
View Answer
Explanation: Silver(I) oxide in aqueous ammonia (“Tollens reagent”) is a mild oxidizing agent which oxidizes aldehydes to carboxylic acids without reacting with carbon-carbon double bonds or many other functional groups.
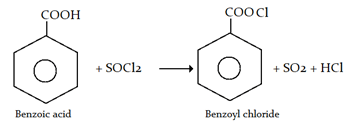
23. Which of the following is used to prepare Benzoyl chloride from benzoic acid?
a) Cl2, H2O
b) SOCl2
c) SO2, Cl2
d) Cl2, hv
View Answer
24. Which of the following compound is yielded from the reduction of nitroalkanes?
a) Amine
b) Acid
c) Diazo compounds
d) Alcohol
View Answer
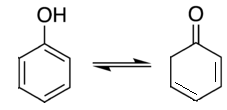
25. Which of the following is a tautomer of phenol?
a) 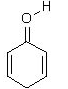
b) 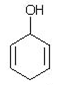
c) 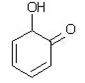
d) 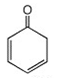
View Answer
26. Which of the following organic compound is formed when aniline reacts with acetaldehyde?
a) Diazonium salt
b) Immine
c) Schiff’s base
d) Carbylamine
View Answer
27. What is the complete IUPAC name of the following substance?
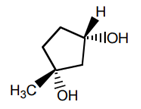
a) (1R,3S)-1-methylcyclopentane-1,3-diol
b) (1S,3R)-1-methylcyclopentane-1,3-diol
c) (1S,3S)-1-methylcyclopentane-1,3-diol
d) (1R,3R)-1-methylcyclopentane-1,3-diol
View Answer
Explanation: The IUPAC name will be (1R,3S)-1-methylcyclopentane-1,3-diol, where 1 position shows R and 3 position shows S.
28. Which of the following is neither an acid nor base?
a) KCl
b) CH3OH
c) HCl
d) CH3COOH
View Answer
Explanation: CH3COOH and CH3OH are organic acids, HCl is a strong acid and KCl is a salt.
29. In which of the following reactions lead tetraacetate is used to cleave a carbon-carbon bond in a glycol?
a) Swern oxidation
b) Criegee oxidation
c) Jones oxidation
d) Baeyer-Villiger Oxidation
View Answer
Explanation: The oxidation of 1,2‐diols (or glycols) to aldehydes or ketones with lead tetraacetate [Pb(OAc)4, LTA] via the cleavage of C‐C bond between the hydroxyl‐carrying carbon atoms is generally known as Criegee Glycol oxidation. This reaction has been reported to occur only in an anhydrous organic solvent.
30. Which of the following compound is used to depress the Central nervous system?
a) Iodoform
b) DDT
c) Freon
d) Chloroform
View Answer
Explanation: Acute chloroform intoxication can cause depression of the central nervous system and may lead to death from lethal arrhythmias or respiratory arrest. Thus, the organic solvent is no longer in clinical use as an anesthetic, but still plays a role in cases of suicide, homicide, or inhalation for psychotropic effects.
Chapterwise Multiple Choice Questions on Organic Chemistry

- Organic Compound Basics
- Hydrocarbons
- Organic Concepts
- Halogen Containing Organic Compounds
- Oxygen Containing Organic Compounds
- Nitrogen Containing Organic Compounds
- Stereochemistry
- Heterocyclic Compounds
- Polymerisation & Biomolecules
1. MCQ on Organic Compound Basics
The section contains Organic Chemistry multiple choice questions and answers on chemical bond, orbitals, physical properties, isomerism, acids and bases.
|
|
|
2. Organic Chemistry MCQ on Hydrocarbons
The section contains Organic Chemistry questions and answers on hydrocarbons, chlorination, nomenclature, resonance, organic reactions, quantitative analysis, alkanes, alkenes, alkynes, alkadienes and aromatic hydrocarbons.
3. Organic Chemistry Multiple Choice Questions on Organic Concepts
The section contains Organic Chemistry MCQs on displacement and nucleophilic substitution reactions, organic compound mechanism, elimination reactions, nuclear magnetic resonance, mass, uv – visible and infrared Spectroscopy, redox reactions, organometallic compounds, photochemistry, nucleophiles, electrophiles, bond cleavage, reaction intermediates and kinetics.
4. Organic Chemistry MCQ on Halogen Containing Organic Compounds
The section contains Organic Chemistry multiple choice questions and answers on alkyl and aryl halides, polyhalogen derivatives, grignard reagent, allyl & vinylic halides.
|
|
|
5. Organic Chemistry Multiple Choice Questions on Oxygen Containing Organic Compounds
The section contains Organic Chemistry questions and answers on preparation and reactions of alcohols, phenols, ethers, oxiranes, glycols, glycerol, aldehydes, ketones, benzaldehyde. aldehydes, tautomerism, physical and chemical properties of carboxylic acids, aldehydes, ketones, acid chlorides and anhydrides, amides, esters, saponification, claisen and cross claisen condensation.
6. Organic Chemistry MCQ on Nitrogen Containing Organic Compounds
The section contains Organic Chemistry MCQs on enamines, physical and chemical properties of Amines, aromatic amines preparation and properties, benzene diazonium chloride, preparation of amines and nitro compounds.
7. Organic Chemistry MCQ on Stereochemistry
The section contains Organic Chemistry multiple choice questions and answers on stereochemistry, stereoisomers, polarimeter, enantiomerism, chilarity, enantiomers, diastereomers and meso structures.
|
|
|
8. Organic Chemistry MCQ on Heterocyclic Compounds
The section contains Organic Chemistry questions and answers on five membered rings, structure and source of pyridine compounds, electrophilic and nucleophilc substitution in pyridine.
|
|
|
9. Organic Chemistry MCQ on Polymerisation & Biomolecules
The section contains Organic Chemistry MCQs on macromolecules, radical vinyl polymerisation, copolymerisation, coordination and step reaction polymerisation, amino acids, dna and rna.
|
|
|
Wish you the best in your endeavor to learn and master Organic Chemistry!