This set of Class 12 Chemistry Chapter 10 Multiple Choice Questions & Answers (MCQs) focuses on “Haloalkanes and Haloarenes”. These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation.
1. Monohalo, dihalo, trihalo and tetrahalo are types of haloalkanes and haloarenes based on the ______
a) type of halogen atom
b) number of halogen atoms
c) nature of carbon atom
d) hybridisation of C atom to which halogen is bonded
View Answer
Explanation: Haloalkanes may be classified as mono, di, tri, tetra and so on depending on the number of halogen atoms present in their structure.
2. A monohaloarene is an example of a/an __________
a) aliphatic halogen compound
b) aromatic halogen compound
c) alkyl halide
d) side chain substituted aryl halide
View Answer
Explanation: A monohaloarene is a compound in which the halogen is directly attached to the benzene ring. Side chain substituted aryl halides are also aromatic halogen compounds with the halogen not directly attached to the benzene ring.
3. What is the general formula for haloalkanes? (X=halogen atom, n = 1, 2, 3…)
a) CnH2nX
b) CnH2n+1X
c) CnH2n-1X
d) CnH2n-3X
View Answer
Explanation: The general formula for haloalkanes is CnH2n+1X where X is a halogen atom and n = 1, 2, 3… The formulae CnH2n-1X and CnH2n-3X are that of haloalkenes and haloalkynes respectively.
4. Which of the following compounds contains an allylic carbon?
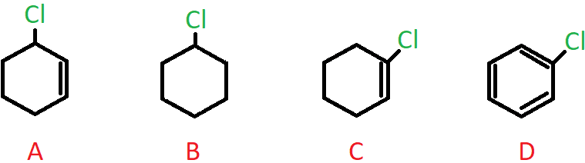
a) A
b) B
c) C
d) D
View Answer
Explanation: A sp3 hybridised C atom present adjacent to a C-C double bond is called an allylic carbon, and when the halogen atom is bonded to this carbon, it is called an allylic halide.
5. Which of the following categories does the compound shown belong to?
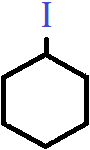
a) Primary haloalkane
b) Secondary haloalkane
c) Tertiary haloalkane
d) Haloarene
View Answer
Explanation: The compound shown is cyclohexyl iodide, in which the halogen atom is bonded to an alkyl group that is alicyclic in nature. Since there are effectively two alkyl groups attached to the carbon bonded to the halogen atom, it is classified as secondary or 2° cyclo alkyl halide.
6. What is the nature of the circled C atom in the following compound?
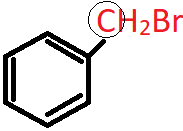
a) sp2 hybridised
b) allylic
c) benzylic
d) vinylic
View Answer
Explanation: The circled C atom is sp3 hybridised and is attached directly to an aromatic ring, hence it is a benzylic carbon and the compound is a 1o benzylic halide.
7. In which of the following cases will the compound be a tertiary (3°) halogen compound? (X=halogen atom)
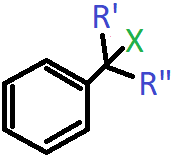
a) R’=R”=H
b) R’=CH3, R”=H
c) R’=H, R”=CH3
d) R’=R”=CH3
View Answer
Explanation: When R and R’ both are CH3 groups, then the carbon atom bonded to the halogen will have three alkyl groups attached to it including the benzene ring.
8. Which of the following is a vinylic halide?
a) CH2=CHCHCl2
b) CH3CHClCH3
c) (CH3)2C=CHCH2Cl
d) CH3CH=CClCH2CH3
View Answer
Explanation: In CH3CH=CClCH2CH3, the Cl is bonded directly to the C atom of a C-C double bond, and hence it is a vinylic halide. CH3CHClCH3 is an alkyl halide whereas CH2=CHCHCl2 and (CH3)2C=CHCH2Cl are allylic halides.
9. The compound C6H5F is an example of a ________ halide.
a) allylic
b) benzylic
c) vinylic
d) aryl
View Answer
Explanation: In C6H5F, the F atom is directly attached to the sp2 hybridised carbon atom of an aromatic ring, i.e., benzene.
10. The compound in which a CH2Br group is attached to a benzene ring is an aryl halide.
a) True
b) False
View Answer
Explanation: The Br atom is bonded to a carbon atom which is attached to sp2 hybridised carbon of benzene. This is an example of a side chain substituted aryl halide.
More MCQs on Class 12 Chemistry Chapter 10:
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 2)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 3)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 4)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 5)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 6)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 7)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 8)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 9)
- Chapter 10 – Haloalkanes and Haloarenes MCQ (Set 10)
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
