This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Preparation Method of Diazonium Salts”.
1. What is the most suitable temperature for the diazotisation reaction to take place?
a) 0°C
b) 10°C
c) 22°C
d) 30°C
View Answer
Explanation: Benzene diazonium salts are prepared by the reaction of aniline with nitrous acid at very low temperatures (273-278K). If the temperature was to cross 278K, phenol will be formed instead.
2. Identify the diazonium group in the diazonium salt ArN2+Br-.
a) ArN2+
b) N2+Br–
c) N2+
d) ArN2Br
View Answer
Explanation: The Ar represents the aromatic ring and Br– is the negative ion that forms the salt by combining with the diazonium group, N2+.
3. What is the correct name of the compound C6H5N2+HSO4–?
a) Benzenediazonium hydrogensulphate
b) Benzenehygrogensulphate diazonium
c) Diazonium benzenehydrogensulphate
d) Hydrogensulphate diazoniumbenzene
View Answer
Explanation: Diazonium salts are named by suffixing diazonium to the parent hydrocarbon (benzene) from which they are formed, followed by the name of the anion (HSO4–).
4. Identify the reagent ‘X’ in the following reaction.
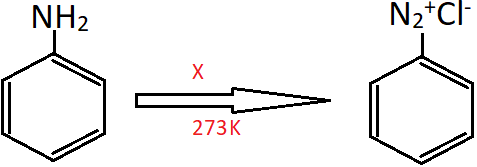
a) HNO3
b) NaNO2 and HCl
c) NaCl and HNO3
d) NaNO2 and H2SO4
View Answer
Explanation: Aniline actually reacts with HNO2 (nitrous acid) at 273K to form Benzenediazonium chloride. But this nitrous acid in produced in situ, by a mixture of NaNO2 and HCl.
5. Why is the reason for the stability of aromatic diazonium salts?
a) Dispersal of negative charge over the benzene ring
b) Dispersal of positive charge over benzene ring
c) Bond between diazonium group and anion
d) High electronegativity of anion compared to the N atom
View Answer
Explanation: The stability of benzenediazonium salt is due to its resonance structures. In three of the resonance structures, the positive charge is dispersed over the benzene ring at ortho and para positions.
6. Aliphatic diazonium salts are highly unstable.
a) True
b) False
View Answer
Explanation: Alkyldiazonium salts are highly unstable as they readily decompose at low temperatures forming a carbocation and nitrogen gas.
7. The preparation of diazonium salts from primary aromatic amines is known as ______
a) acylation
b) alkylation
c) benzonation
d) diazotisation
View Answer
Explanation: This is a method of preparation of diazonium salts. This occurs by the formation of nitrosonium ion followed by diazohydroxide, which on protonation gives diazonium ion. This takes up the acid anion to form diazonium salt.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Mathematics MCQs
- Check Class 12 - Chemistry Books
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Biology MCQs
