This set of Class 12 Chemistry Chapter 15 Multiple Choice Questions & Answers (MCQs) focuses on “Types of Polymerisation Reactions – 2”.
1. What is the polymer obtained from the condensation of NH2-(CH2)6-NH2 and COOH-(CH2)8-COOH?
a) Nylon-6,6
b) Nylon-6
c) Nylon-6,10
d) Terylene
View Answer
Explanation: Nylons are polyamides obtained from the condensation between amines and dicarboxylic acids. The have amide linkages (NHCO). Hexamethylenediamine and sebacic acid from the polymer nylon-6,10.
2. Cyclohexanone on treatment with hydroxylamine gives P, which on treatment with sulphuric acid gives Q. When Q is heated with water at high temperature, R is obtained. Identify R.
a) Nylon-6
b) Caprolactam
c) Amino caproic acid
d) Nylon-6,6
View Answer
Explanation: Nylon-6 is the polymer of caprolactam (Q), which is obtained from the oxidation of cyclohexane to give cyclohexanone(P), followed by treatment with H2SO4. Caprolactam on heating with water gives amino caproic acid which polymerises to nylon-6.
3. Glyptal is obtained from the condensation polymerisation of ethylene glycol with _______
a) terephthalic acid
b) phthalic acid
c) isophthalic acid
d) trimesic acid
View Answer
Explanation: Glyptal is a polyester (containing COO linkages) obtained from the polycondensation reaction of ethane-1,2-diol and benzene-1,2-dicarboxylic acid (phthalic acid). It is cross-linked polymer.
4. Which of the following is not required for the production of dacron?
a) 450K temperature
b) Ethylene glycol
c) Terephthalic acid
d) Triethylaluminium-titanium tetrachloride
View Answer
Explanation: Dacron or terylene is a polyester of ethylene glycol and terephthalic acid. It is produced by heating the two at 420-460K and in the presence of zinc acetate-antimony trioxide, which acts as a catalyst. On the other hand, triethylaluminium-tetrachloride is used in the synthesis of HDPE.
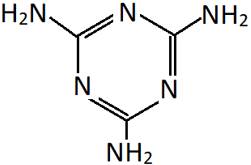
5. Identify the polymer generated by the reaction of methanal with the shown compound.
a) Melamine-formaldehyde
b) Novolac
c) Bakelite
d) Phenol-formaldehyde
View Answer
Explanation: The compound shown is a heterocyclic triamine called melamine. It reacts with methanal to form a resin intermediate which polymerises to form a melamine-formaldehyde polymer.
6. Bakelite is a cross-linked polymer.
a) True
b) False
View Answer
Explanation: Phenol-formaldehyde condensation initially forms a linear polymer, Novolac. This on further heating with formaldehyde undergoes cross-linking through CH2 groups to form bakelite.
7. Which of the following phenols are able to form Novolac?
a) m-Hydroxybenzyl alcohol
b) o-Hydroxybenzyl alcohol and p-hydroxybenzyl alcohol
c) 2,4-(Dihydroxymethyl)phenol
d) 2,4,6-(Trihydroxymethyl)phenol
View Answer
Explanation: Novolac is a linear chain phenol-formaldehyde polymer. The ortho and para hydroxy derivatives of benzyl alcohol, initially formed by the reaction of phenol with HCHO, further react with phenol to form compounds having benzene rings joined together by -CH2– links (Novolac).
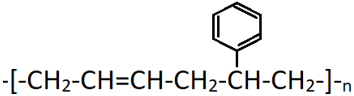
8. The compound shown is a ________
a) chain growth copolymer
b) step growth copolymer
c) chain growth homopolymer
d) step growth homopolymer
View Answer
Explanation: The polymer shown is butadiene-styrene copolymer which is formed by the addition polymerisation of two different unsaturated compounds, 1,3-butadiene and styrene. It is tough and used as a substitute for natural rubber.
9. The polymer of type [-AAAABBBAAAABBB-]n, where A and B are two different monomers, is called a ______ copolymer.
a) alternating
b) block
c) graft
d) random
View Answer
Explanation: Bock copolymers are those in which blocks of each type of monomer alternate with each other. In this case, the block AAAA and block BBB are alternatively repeated after each other.
10. Which of the following is the monomer of natural rubber?
a) Neoprene
b) Isoprene
c) Chloroprene
d) Butadiene
View Answer
Explanation: Isoprene (or 2-methyl-1,3-butadiene) repeats itself in a linear chain to form natural rubber. This is also called as cis-1,4-polyisoprene, in which the monomers are linked by weak van der Waals forces, thus making it elastic in nature.
11. Which of the following is incorrect regarding natural rubber?
a) Shows thermoplastic behaviour
b) Soluble in benzene
c) Resistant to attack by oxidising agents
d) Shows high water absorption capacity
View Answer
Explanation: Natural rubber becomes soft at temperatures above 335K and shows brittle character at temperatures below 283K. Hence, it shows thermoplastic behaviour. It is soluble in non-polar solvents like benzene, toluene, etc. and is not susceptible to attack by oxidising agents.
12. Vulcanization is carried out with which element?
a) Sulphur
b) Phosphorous
c) Oxygen
d) Chlorine
View Answer
Explanation: Vulcanization is a process of improving the physical properties of natural rubber by introducing sulphur cross-links at the reactive sites of double bonds. This is done by heating the rubber with sulphur and an additive.
13. X on polymerisation gives neoprene. Identify X.
a) 1-Chloro-1,3-butadiene
b) 2-Chloro-1,3-butadiene
c) 1-Methyl-1,3-butadiene
d) 2-Methyl-1,3-butadiene
View Answer
Explanation: Neoprene or polyisoprene is a synthetic rubber obtained from the addition polymerisation of chloroprene (2-chloro-1,3-butadiene) through free radical mechanism. It has a better stability towards oxidation than natural rubber.
14. Buna-N involves acrylonitrile as one of the monomers.
a) True
b) False
View Answer
Explanation: Buna-N is a nitrile rubber formed by the copolymerisation of 1,3-butadiene and acrylonitrile. On the other hand, buna-S is a styrene rubber, with vinylbenzene as one monomer in place of acrylonitrile.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Mathematics MCQs
