This set of Class 12 Chemistry Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Nomenclature and Structure of Carbonyl Groups – 2”.
1. Acetone is the common name of which of the following ketones?
a) Dimethyl ketone
b) Ethyl methyl ketone
c) Diethyl ketone
d) Methyl n-propyl ketone
View Answer
Explanation: In the common system, the ketones are named by listing the alkyl groups present in it in alphabetical order. The simplest dimethyl ketone is also known as acetone.
2. Which of the following will be the main group when present in a compound together?
a) -CHO
b) -CO
c) -OH
d) -Cl
View Answer
Explanation: If a compound contains both aldehyde and ketone groups, then the former is considered as the principal functional group, and the ketone is regarded as substituent. Thus, it is named as a derivative of alkanal.
3. _________ ketones are generally named by adding the name of the acyl group as prefix to the word phenone.
a) Ethyl methyl
b) Dialkyl
c) Alkyl phenyl
d) Diphenyl
View Answer
Explanation: For example, the ketone with COCH3 group attached to a benzene ring is named as acetophenone. Also, diphenyl ketone as benzophenone.
4. What is the IUPAC name of CH3-CO-CH2-CH2-CH3?
a) Butan-1-one
b) Butan-2-one
c) Pentan-1-one
d) Pentan-2-one
View Answer
Explanation: The carbon of the CO ketonic group should be included in the parent chain, and the numbering starts from the end nearer to the carbonyl group. Hence in the given compound, there are five carbon atoms in the parent chain and the carbonyl carbon is second one.
5. What is the name of the shown compound?
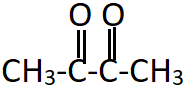
a) Ethane-1,2-dione
b) Butane-1,2-dione
c) Butanone
d) Butane-2,3-dione
View Answer
Explanation: This is a symmetrical dimethyl diketone. There are four carbons in the parent chain, including the two carbons of ketonic group and numbering can start from either end of the chain.
6. What is the correct IUPAC name of α-Methylcyclohexanone?
a) Methylcyclohexanone
b) Methylcyclohexan-2-one
c) 2-Methylcyclohexanone
d) Cyclomethylhexan-2-one
View Answer
Explanation: Alpha represents the presence of a substituent on the carbon next to the carbon of the CO group. The ketone group is given the lower number in case of substituted ketones hence the number 2 is associated with the methyl group in this case.
7. What is the IUPAC name of diisopropyl ketone?
a) 1,3-Diisopropylpropan-2-one
b) 2,4-Dimethylpentan-3-one
c) 2-Methyl-1-(1-methylethyl)propan-1-one
d) 1,3-Dimethylpropan-2-one
View Answer
Explanation: The formula of diisopropyl ketone is (CH3)2CHCOCH(CH3)2 which consists of 7 carbon atoms. The longest parent chain including the keto group is of 5 carbon atoms, leaving out one C atom from the methyl group at each end. This is a symmetric ketone and the methyl groups are present at the second and fourth carbon starting from any one end and the C-O double bond is present at the third carbon.
8. Identify the correct IUPAC name of the following compound.
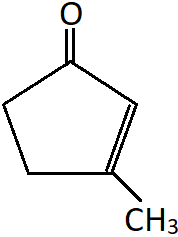
a) 3-Methylcyclopent-2-en-1-one
b) 1-Methylcyclopent-1-en-3-one
c) Cyclo-1-methyl-pent-1-en-3-one
d) Cyclo-3-methyl-pent-2-en-1-one
View Answer
Explanation: This is a cyclic ketone with a double bond and a substituted methyl group. In this compound the keto carbon will be given the lowest number. Hence, the double bond is at second carbon and methyl group at the third carbon. It is named as a substituted cyclopentanone.
9. What is the IUPAC name of the following compound?
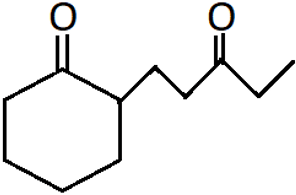
a) 6-(2-oxocyclohexyl)-hexan-3-one
b) 2-(3-oxopentyl)-cyclohexan-1-one
c) 2-(4-oxopentyl)-cyclohexan-1-one
d) 5-(2-oxocyclohexyl)-hexan-3-one
View Answer
Explanation: The cyclic ketone is considered as the main group with the 3-oxopentyl keto group substituted at alpha carbon.
10. What is the approximate planar bond angle between the carbonyl C-O bond and the bond between the carbonyl carbon and the atom attached to it?
a) 60°
b) 90°
c) 120°
d) 135°
View Answer
Explanation: The bond angles associated with the carbonyl group is approximately 120°, which is similar to that of a trigonal coplanar structure.
11. The C-O bond in carbonyls is polarised, and hence the carbonyl carbon and carbonyl oxygen act as _________ and ________ respectively.
a) electrophile; nucleophile
b) nucleophile; electrophile
c) Lewis base; Lewis acid
d) electrophile; Lewis acid
View Answer
Explanation: The polarity of the C-O bond is due to the higher electronegativity of O than C. This means that oxygen is a nucleophilic centre and carbon is an electron seeking centre.
12. Ketones are more polar than ethers.
a) True
b) False
View Answer
Explanation: Ketones are carbonyl compounds which show neutral as well as dipolar resonance structures, which is not possible in ethers.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
