This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Amines Chemical Reactions – 4”.
1. Which gas is produced when ethanamine reacts with nitrous acid?
a) N2
b) H2
c) HCl
d) O2
View Answer
Explanation: Nitrogen gas is evolved due to the decomposition of diazonium salts which are formed from the reaction of 1° aliphatic amines with HNO2. This is used in the estimation of amino acids and proteins.
2. What is the main product of the following reaction?
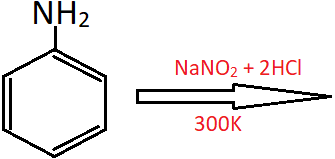
a) Benzene diazonium chloride
b) Phenol
c) o-Nitrosoaniline
d) p-Nitrosoaniline
View Answer
Explanation: Primary aromatic amines react with nitrous acid at cold temperatures (273-278K) to form diazonium salts. However, if the temperature is more than 278K, phenol is formed along with the evolution of nitrogen gas.
3. Tertiary amines dissolve in nitrous acid to form corresponding salts.
a) True
b) False
View Answer
Explanation: Tertiary amines on reaction with cold HNO2 remain dissolved, forming amine nitrite salts, which decompose to nitrosoamines and alcohol on warming.
4. Which of the following is not a final product of the reaction between propylamine and nitrous acid?
a) CH3CH2CH2N2Cl
b) CH3CH2CH2OH
c) N2 gas
d) H2O
View Answer
Explanation: CH3CH2CH2N2Cl is a diazonium salt derivative of propanamine and is first formed when it reacts with HNO2. This is a very unstable compound and immediately decomposes to give propanol and evolves nitrogen gas.
5. Diethylamine reacts with nitrous acid in the cold to form _______
a) diazonium salt
b) alcohol
c) imine
d) nitrosoamine
View Answer
Explanation: Secondary amines react slowly with nitrous acid at low temperatures to give yellow oily nitrosoamines. Diethylamine specifically produces N,N-diethylnitrosoamine.
6. Hinsberg’s reagent is _______
a) benzenesulfonic acid
b) benzenesulphonyl chloride
c) p-toluenesulphonyl chloride
d) chlorosulphuric acid
View Answer
Explanation: Benzenesulphonyl chloride (C6H5SO2Cl), or arylsulphonyl chloride, is known as Hinsberg’s reagent. It is an important compound used in the distinction of different classes of amines.
7. Which of the following amines, on reaction with benzenesulphonyl chloride, will give a sulphonamide that is insoluble in alkali?
a) Ethylamine
b) Ethylmethylamine
c) Trimethylamine
d) Aniline
View Answer
Explanation: Secondary amines on reaction with Hinsberg’s reagent produce sulphonamides without any hydrogen atom attached to the nitrogen atom. Hence, it is not acidic and therefore insoluble in alkali. The amide produced in this case is N-ethyl-N-methylbenzenesulphonamide.
8. Which of the following amines will form a product that is soluble in KOH, on reaction with Hinsberg’s reagent?
a) Isopropylamine
b) Diethylamine
c) N,N-Dimethylpropylamine
d) N,N-Dimethylaniline
View Answer
Explanation: The reaction of Hinsberg’s reagent with Isopropylamine gives N-isopropylbenzene sulphonamide. The lone hydrogen attached to the N atom is strongly acidic due to the presence of strong electron withdrawing sulphonyl group. Hence, is it soluble in alkali like KOH.
9. Which of the following reactions/tests does not help in the distinction between ethylamine and diethylamine?
a) Carbylamine test
b) Hinsberg’s test
c) Reaction with HNO2
d) Reaction with CH3CH2Br
View Answer
Explanation: Both ethylamine and diethylamine on reaction with CH3CH2Br eventually gives quaternary ammonium salt. Hence, the alkylation of primary amines cannot be used as a distinction method.
10. Aniline is a _______ directing compound.
a) ortho
b) meta
c) ortho and para
d) ortho, meta and para
View Answer
Explanation: The ortho and para positions with respect to NH2 group in aniline are centres of high electron density. This is due to the resonance structures of aniline. Thus, NH2 is ortho and para directing and a powerful activating group.
11. The activating effect of -NHCOCH3 group is ______ as compared to -NH2 group.
a) less
b) same
c) more
d) very high
View Answer
Explanation: The -NHCOCH3 group forms two resonance structures, where the lone pair of nitrogen interacts with the lone pairs on oxygen atom. This makes the lone electron pair on N less available for donation to benzene ring.
12. What is the major product formed when aniline reacts with bromine water at room temperature?
a) 2-Bromoaniline
b) 4-Bromoanline
c) 2,6-Dibromoaniline
d) 2,4,6-Tribromoaniline
View Answer
Explanation: Since amino group is highly ortho and para directing, it substitutes the Br group at the para as well as both ortho positions (2,4,6) to give a white precipitate of a tribromo substituted aniline.
13. What is the order of quantities of all isomers of nitroaniline formed on the reaction of aniline with nitric acid and sulphuric acid at 288K?
a) ortho > meta > para
b) para > ortho > meta
c) para > meta > ortho
d) meta > para > ortho
View Answer
Explanation: Nitration of aniline produces nitro derivatives. Under controlled conditions, aniline is protonated (by strong acidic medium) to form anilinium ion, which is meta directing. Therefore, apart from p-nitroaniline (51%) and o-nitroaniline (2%), a significant amount of m-nitroaniline (47%) is also formed.
14. It is possible to obtain para isomers of anilines as the major product in electrophilic ring substitution of aniline.
a) True
b) False
View Answer
Explanation: This is possible by protecting the amino group of aniline by reacting it with acetic anhydride (acetylation) to give acetanilide. This compound on desired substitution reaction followed by hydrolysis gives para substituted aniline as major product.
15. A compound ‘P’ on treating with concentrated H2SO4 forms ‘R’. The product ‘R’ on heating at 460K forms a zwitter ionic compound. Identify P, R respectively.
a) Aniline; anilinium hydrogensulphate
b) Aniline; sulphanilic acid
c) Anilinium hydrogensulphate; sulphanilic acid
d) Sulphanilic acid; anilinium hydrogensulphate
View Answer
Explanation: Aniline (P) reacts with conc. H2SO4 to form anilinium hydrogensulphate (R) which on heating at 453-473K produces sulphanilic acid (zwitter ionic compound).
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Biology MCQs
- Check Class 12 - Chemistry Books
