This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Preparation of Amines – 1”.
1. Which of the following is the most preferred reagent for reducing nitroethane to ethylamine?
a) H2/Pt
b) Sn/HCl
c) Fe/HCl
d) Zn/HCl
View Answer
Explanation: Reduction of nitroalkanes with iron scrap and HCl is preferred because of the formation of FeCl2 which gets hydrolysed to release HCl during the reaction. Thus, only a small amount of HCl is required to initiate the reaction.
2. Which of the following reagents cannot be used to convert nitrobenzene to aniline?
a) LiAlH4-dry ether
b) SnCl2/HCl
c) H2/Pd-ethanol
d) Zn in HCl
View Answer
Explanation: LiAlH4 may be used for the reduction of aliphatic nitro compounds to respective amines. But with aromatic nitro compounds (nitrobenzene), it gives azobenzene and not primary amines (aniline).
3. What compound is formed when hydrogen gas is passed through nitrobenzene in the presence of finely divided nickel?
a) Aniline
b) 2-Nitroaniline
c) 3-Nitroaniline
d) 4-Nitroaniline
View Answer
Explanation: The nitro (NO2) group of nitrobenzene is reduced to amino (NH2) group by the replacement of two oxygens by two hydrogen to give aniline or benzenamine.
4. How many water molecules are formed as the by product of reduction of one molecule of nitropropane to one molecule of propanamine, with hydrogen gas in Pt catalyst?
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: One molecule of nitropropane reacts with 3 molecules of hydrogen gas (H2) in the presence of Ni/Pt/Pd catalyst to form one molecule of propanamine along with two molecules of water.
5. Ammonolysis is a type of _________ reaction.
a) electrophilic addition
b) electrophilic substitution
c) nucleophilic addition
d) nucleophilic substitution
View Answer
Explanation: Alkyl and benzyl halides on reaction with an alcoholic solution of ammonia (nucleophile) undergoes nucleophilic substitution of the halogen atom by the amino group (-NH2). This process is known as ammonolysis.
6. What is the type of amine obtained from the ammonolysis of alkyl halides?
a) Primary
b) Primary and secondary
c) Secondary and tertiary
d) Primary, secondary and tertiary
View Answer
Explanation: When an alcoholic solution of ammonia is heated with alkyl halides, a mixture of all three types of amines, i.e., primary, secondary and tertiary amines are formed along with a quaternary ammonium salt.
7. What is the most suitable condition for the ammonolysis of an alkyl halide?
a) 273K, open tube
b) 273K, sealed tube
c) 373K, open tube
d) 373K, sealed tube
View Answer
Explanation: The cleavage of the carbon-halogen bond (of the alkyl halide) by a nucleophile takes place under high pressure and temperature. Thus, the ammonolysis is carried out in a sealed tube at about 100°C.
8. What is obtained when the following compound undergoes ammonolysis in a sealed tube at 373K with iodomethane?
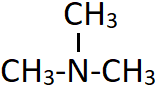
a) Dimethylamine
b) N-Iodo-N-methylmethanamine
c) Tetramethyl ammonium iodide
d) No reaction
View Answer
Explanation: Tertiary amines are formed as the ammonolysis of alkyl halides proceeds to form 1° and 2° amines, which further reacts with the alkyl halide. The compound shown is trimethylamine which reacts with iodomethane (alkyl halide) to form a quaternary ammonium salt [(CH3)4N+]I–.
9. Aniline can be formed from the ammonolysis of chlorobenzene.
a) True
b) False
View Answer
Explanation: Ammonolysis is not suitable for the production of aryl amines, because aryl halides are stable and relatively less reactive toward nucleophilic substitution than alkyl halides. Since chlorobenzene is an aryl halide, it does not undergo ammonolysis to form aniline.
10. The main product formed when the ammonolysis is carried out using excess of iodoethane is _______
a) 1° amine
b) 2° amine
c) 3° amine
d) quaternary ammonium salt
View Answer
Explanation: When an excess of alkyl halide is used, the presence of a base will allow the excess hydrogen halide formed to be consumed, resulting in the formation of quaternary ammonium salt as a major product.
11. What is the correct order of reactivity of the following alkyl halides towards ammonolysis reaction?
a) CH3I > CH3Br > CH3Cl
b) CH3I > CH3Cl > CH3Br
c) CH3Cl > CH3Br > CH3I
d) CH3Br > CH3Cl > CH3I
View Answer
Explanation: Iodine is the most electropositive atom of chlorine and bromine halogens. This makes it more susceptible to a nucleophilic attack than towards Cl or Br. Hence, CH3I is more reactive towards ammonolysis.
12. Ammonolysis is a reaction between an alkyl halide and most preferably an ______ solution of NH3.
a) alkaline
b) acidic
c) alcoholic
d) aqueous
View Answer
Explanation: An alcoholic medium promotes the occurrence of a nucleophilic attack of NH3 molecule on the alkyl/benzyl halide. Generally, ethanol is used for this purpose.
13. Identify the product B in the shown reaction.
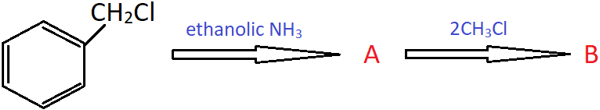
a) Benzylamine
b) N-Methylphenylmethanamine
c) N,N-Dimethylphenylmethanamine
d) N,N-Dichlorophenylmethanamine
View Answer
Explanation: Benzyl chloride reacts with ethanolic ammonia to form benzylamine (A) which reacts with one molecule of CH3Cl to give N-methyphenylmethanamine, which further reacts with one more molecule of CH3Cl to give N, N-dimethylphenylmethanamine.
14. Only primary amine is formed as the main product when ammonolysis of an alkyl halide takes place with large excess of ammonia.
a) True
b) False
View Answer
Explanation: When NH3 is taken in excess in the reaction mixture, the alkyl halide is more likely to react with an NH3 molecule rather than with a molecule of amine which is present in smaller amount. Therefore, only primary amine is formed as the main product.
15. Which of the following would not be a good choice for reducing an aryl nitro compound to an amine?
a) H2/Pt
b) LiAlH4-ether
c) Fe and HCl
d) Sn and HCl
View Answer
Explanation: Lithium aluminium hydride is a reducing agent but does not react with nitro compounds. Instead it reduces nitriles to give corresponding primary amines.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
