This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Amines”. These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation.
1. When two alkyl groups are attached to the nitrogen atom in an amine, it is known as a _______ amine.
a) primary
b) secondary
c) tertiary
d) aromatic
View Answer
Explanation: Amines are classified as secondary or 2° when two of the hydrogen atoms of ammonia are replaced by an alkyl group and the third H remains attached as it is.
2. When only two hydrogen atoms are attached to the nitrogen of an amine, it is classified as a ________
amine.
a) primary
b) secondary
c) aliphatic
d) aromatic
View Answer
Explanation: When an amine has two hydrogen atoms individually bonded to the nitrogen, it means that the third group is an alkyl or aryl substituent. This is called as a primary or 1° amine as only one H atom is replaced.
3. Which of the following is not a classification of amines?
a) Primary
b) Secondary
c) Tertiary
d) Quaternary
View Answer
Explanation: Amines may be classified as primary, secondary or tertiary depending on whether 1, 2 or 3 hydrogen atoms of NH3 are replaced by alkyl/aryl groups respectively. Quaternary ammonium compounds are a different class of compounds where all four hydrogen atoms of ammonium salts are replaced by alkyl/aryl groups.
4. What is the most suitable classification of the following amine?
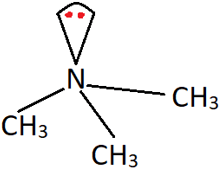
a) Secondary amine
b) Dialkyl amine
c) Tertiary amine
d) Aromatic amine
View Answer
Explanation: This is trimethylamine, in which all the three hydrogen atoms are replaced by methyl (CH3) groups. Hence, it is a tertiary or 3° amine.
5. Which of the following is a 2° amine?
a) CH3CH2NHCH3
b) (CH3)3N
c) (CH3CH2CH2)2NCH3
d) C6H5NH2
View Answer
Explanation: The three groups attached to CH3CH2NHCH3 are one methyl group, one ethyl group and one hydrogen atom. This means that two of the hydrogen atoms are replaced and hence, it is a 2° or secondary amine.
6. Benzylamine is an aryl amine.
a) True
b) False
View Answer
Explanation: Aryl amines are aromatic amines in which the nitrogen atom is directly bonded to the benzene ring. Whereas in benzylamine (C6H5CH2NH2), the nitrogen is attached to the side chain of the aromatic ring. Hence, it is an arylalkyl amine.
7. Which of the following is the most suitable classification for C6H5NHCH3?
a) Tertiary amine
b) Aliphatic amine
c) Arylalkyl amine
d) Mixed amine
View Answer
Explanation: In the given amine, there are two substituents, one methyl group and one phenyl group. Hence, it is a secondary aryl amine and since both the substituent groups are different, it is a mixed amine.
8. Which of the following is an arylalkyl amine?
a) C6H5NHC6H5
b) C6H5NH2
c) (C6H5CH2)NH
d) (C6H5)3N
View Answer
Explanation: Arylalkyl amines are aromatic amines which are side chain substituted, i.e., the nitrogen is not directly attached to the phenyl group but to a side chain of the benzene ring. In (C6H5CH2)NH, there are two same groups attached to the nitrogen where it is attached to a benzyl carbon, instead of an aryl carbon.
9. Which of the following categories does CH3-NH-CH3 not belong to?
a) Secondary amine
b) Simple amine
c) Aliphatic amine
d) Mixed amine
View Answer
Explanation: There are two hydrogen that replaced by CH3 group, making it a secondary amine and since both the replacing groups are identical, it is a simple amine. Also, it is aliphatic in nature as both the substituents are alkyl groups.
10. Aromatic amines cannot have an alkyl group attached to N atom.
a) True
b) False
View Answer
Explanation: There can be an alkyl group attached to an aromatic amine, but at least one of the substituents should be a phenyl group in aryl amine. For arylalkyl amines (which are also aromatic), the benzene ring is not directly linked to the nitrogen.
11. (C3H7)N(CH3)(C2H5) is not a ________ amine.
a) secondary
b) tertiary
c) aliphatic
d) mixed
View Answer
Explanation: In the given amine, all the three hydrogens of NH3 are replaced by one each of propyl, ethyl and methyl groups, and is a tertiary or 3° amine. Since all the three groups are alkyl and different, it is an aliphatic mixed amine.
12. The compound shown is a _______ amine.
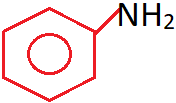
a) 1° aryl
b) 1° arylalkyl
c) 2° aryl
d) 2° arylalkyl
View Answer
Explanation: Only one hydrogen atom is replaced by an aromatic group, in which the nitrogen is directly linked to the sp2 hybridised carbon of benzene ring. Hence, it is a primary aromatic aryl amine.
13. Which of the following best describes the amine (CH3CH2)3N?
a) Secondary, simple
b) Secondary, mixed
c) Tertiary, simple
d) Tertiary, mixed
View Answer
Explanation: All the three hydrogen atoms of ammonia are replaced by the same ethyl group in (CH3CH2)3N, which makes is a 3° simple amine.
14. Which of the following is a primary arylalkyl amine?
a) CH3NH2
b) C6H5CH2NH2
c) C6H5NH2
d) (C6H5)2NH
View Answer
Explanation: Since it should be primary, it should have an alkyl/aryl group attached to the NH2 molecule, and because it is arylalkyl, the nitrogen should be linked to an sp3 hybridised benzyl carbon rather than to an aryl carbon.
15. The compound CH3NHC6H5 is a ______ amine.
a) aliphatic simple
b) aliphatic mixed
c) aromatic simple
d) aromatic mixed
View Answer
Explanation: Since the compound consists of a benzene ring, it invariably is an aromatic amine. Moreover, it also contains an alkyl methyl group beside the phenyl group and this makes it a mixed amine.
More MCQs on Class 12 Chemistry Chapter 13:
- Chapter 13 – Amines MCQ (Set 2)
- Chapter 13 – Amines MCQ (Set 3)
- Chapter 13 – Amines MCQ (Set 4)
- Chapter 13 – Amines MCQ (Set 5)
- Chapter 13 – Amines MCQ (Set 6)
- Chapter 13 – Amines MCQ (Set 7)
- Chapter 13 – Amines MCQ (Set 8)
- Chapter 13 – Amines MCQ (Set 9)
- Chapter 13 – Amines MCQ (Set 10)
- Chapter 13 – Amines MCQ (Set 11)
- Chapter 13 – Amines MCQ (Set 12)
- Chapter 13 – Amines MCQ (Set 13)
- Chapter 13 – Amines MCQ (Set 14)
- Chapter 13 – Amines MCQ (Set 15)
- Chapter 13 – Amines MCQ (Set 16)
- Chapter 13 – Amines MCQ (Set 17)
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Physics MCQs
