This set of Class 12 Chemistry Chapter 9 Multiple Choice Questions & Answers (MCQs) focuses on “Bonding in Metal Carbonyls”.
1. What is the geometry of pentacarbonyliron(0)?
a) Square planar
b) Tetrahedral
c) Trigonal bipyramidal
d) Octahedral
View Answer
Explanation: The coordination number of pentacarbonyliron(0) is 5 as CO is a unidentate ligand and hence its geometry id trigonal bipyramidal.
2. The following structure of a carbonyl compound is formed by which transition metal.
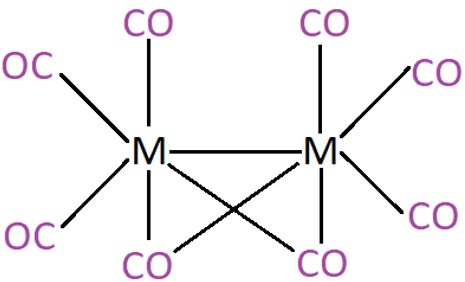
a) Ni
b) Cr
c) Mn
d) Co
View Answer
Explanation: There are 8 carbonyl ligands present. This compound is formed by cobalt metal ion an is named octacarbonyldicobalt(0). It also has Co-Co bond bridged by two carbonyl groups.
3. The metal-carbon bond in metal carbonyls possesses only sigma character.
a) True
b) False
View Answer
Explanation: The M-C bond in metal carbonyls has both sigma and pi bonds and this helps in creating a synergic effect and, hence strengthening the bond.
4. How is the M-C pi bond formed?
a) Donation of electron pair of half-filled metal d orbital to empty bonding pi orbital of CO
b) Donation of electron pair of filled metal d orbital to empty bonding pi orbital of CO
c) Donation of electron pair of filled metal d orbital to empty antibonding pi orbital of CO
d) Donation of electron pair of half-filled metal d orbital to empty antibonding pi orbital of CO
View Answer
Explanation: The pi bond involves donation of electrons from filled metal d orbitals into empty antibonding pi orbitals of CO. This is also called a back bond.
5. The donation of lone pair of electrons of CO carbon into the vacant orbital of metal atom results in _________ bond.
a) sigma
b) pi
c) back
d) synergic
View Answer
Explanation: Synergic bonding is the overall effect of the sigma and pi interactions in metal carbonyl bonds. M-C pi bonds are also known as back bonding.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
