This set of Class 12 Chemistry Chapter 10 Multiple Choice Questions & Answers (MCQs) focuses on “Methods of Preparation of Haloalkanes”.
1. What is the correct order of reactivity of alcohols with a given haloacid?
a) 1° > 2° > 3°
b) 3° > 2° > 1°
c) 1° > 3° > 2°
d) 2° > 1° > 3°
View Answer
Explanation: When more number of electron donating groups are bonded to the C atom attached to the OH group, the polarity of C-OH bond increases. Thus, the reactivity of the alcohol increases.
2. What is the correct order of reactivity of the following haloacids with a given alcohol?
a) HCl > HBr > HI
b) HI > HBr > HCl
c) HBr > HCl > HI
d) HI > HCl > HBr
View Answer
Explanation: The bond dissociation enthalpy of the carbon-halogen bond between H and Cl is the highest and that between H and I is the lowest among HI, HBr and HCl.
3. What is the catalyst in the reaction of a primary alcohol with HCl to obtain a chloroalkane?
a) anhydrous ZnCl2
b) concentrated H2SO4
c) red phosphorous
d) pyridine
View Answer
Explanation: The presence of anhydrous ZnCl2 is to be break the C-O bond in alcohols. ZnCl2 is a Lewis acid and reacts with the oxygen of the alcohol group.
4. The reaction of a primary alcohol with which of the following gives purely a haloalkane?
a) Phosphorus trichloride
b) Phosphorus pentachloride
c) Thionyl chloride
d) Sulphuryl chloride
View Answer
Explanation: The reaction of alcohol with SOCl2 gives a chloroalkane along with gases SO2 and HCl which are easily escapable, leaving behind the pure alkyl chloride.
5. When ethanol reacts with PCl5, it gives three products which include chloroethane and hydrochloric acid. What is the third product?
a) Phosphorus acid
b) Phosphoric acid
c) Phosphorus trichloride
d) Phosphoryl chloride
View Answer
Explanation: The reaction of CH3CH2OH with PCl5 yields CH3CH2Cl, POCl3 (phosphoryl acid) and HCl. This is a method of preparation of chloroalkanes from alcohols.
6. What do you get by heating a mixture of hexanol and concentrated aqueous hydrogen chloride?
a) Cyclochlorohexane
b) Chlorohexane
c) Phosphorus acid
d) No reaction
View Answer
Explanation: Alkyl chlorides can be prepared by heating a mixture of alcohol and concentrated aqueous halogen acid.
7. Iodoalkanes can be obtained by heating alcohols with potassium iodide in __________
a) hypophosphrus acid
b) phosphorus acid
c) phosphoric acid
d) hypophosphoric acid
View Answer
Explanation: When alcohols are heated with potassium or sodium iodide in 95% phosphoric acid (H3PO4), iodoalkanes are generated.
8. The following reaction to form haloalkanes is an example of which type of reaction?
Propane + Cl2 (in the presence of UV light) = 1-Chloropropane + 2-Chloropropane
a) Free radical substitution
b) Halogen exchange
c) Finkelstein reaction
d) Swarts reaction
View Answer
Explanation: Free radical chlorination of alkanes give a mixture of isomeric haloalkanes which are difficult to separate. This reaction takes place in the presence of UV light or heat.
9. How many monohaloalkane isomers can be formed on the free radical bromination of (CH3)2CHCH2CH3?
a) 2
b) 3
c) 4
d) 5
View Answer
Explanation: There are four different types of hydrogen atoms that can be replaced to give the following four isomers; (CH3)2CHCH2CH2Br, (CH3)2CHCH(Br)CH3, (CH3)2C(Br)CH2CH3 and CH3CH(CH2Br)CH2CH3.
10. When propene reacts with HBr, two products are formed out of which one is predominates the other. Identify the minor product.
a) 1-Bromopropane
b) 2-Bromopropane
c) 1-Bromobutane
d) 2-Bromobutane
View Answer
Explanation: According to Markovnikov’s rule, the negative part of the reagent (Br) attaches itself to the C atom with lesser number of hydrogen atoms. Hence, the Br attaches majorly to the second C atom of propane and forms 2-Bromopropane as the major product.
11. Which of the following is incorrect with regard to the reaction between C2H4 and Cl2 in CCl4?
a) It results in the formation of a vicinal dihalide
b) It results in the breaking of the C-C double bond
c) It results in the formation of a colourless compound
d) It results in the discharge of a reddish-brown colour
View Answer
Explanation: The reddish-brown colour is discharged when Br2 reacts with an alkene to form a vic-dihalide. This makes it an important test for the detection of a double bond.
12. The Finkelstein reaction takes place in what medium?
a) Concentrated HCl
b) Concentrated H2SO4
c) Dry acetone
d) Water
View Answer
Explanation: When the alkyl bromide/chloride reacts with sodium iodide in dry acetone, the NaBr or NaCl formed gets precipitated in dry acetone. This facilitates the forward reaction according to Le Chatelier’s Principle.
13. What will be the product of the following reaction?
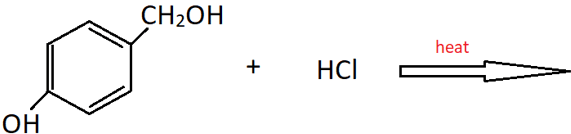
a) 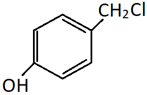
b) 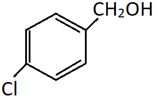
c) 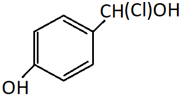
d) 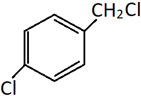
View Answer
Explanation: This is a method of preparation of alkyl chloride from alchol by heating it with HCl. Only the alcoholic OH group will be substituted by Cl. The phenolic OH will reamain as it is.
14. The Finkelstein reaction can be used to prepare alkyl fluorides.
a) True
b) False
View Answer
Explanation: The reagent in Finkelstein reaction is strictly sodium iodide which reacts with either alkyl bromides or chlorides to exchange the iodine with the other halogen group to form alkyl iodides.
15. Identify ‘X’ in the following reaction.
![]()
a) AgF
b) Hg2F2
c) CoF2
d) SbF3
View Answer
Explanation: This is an example of Swarts reaction to prepare alkyl fluorides from alkyl bromides or chlorides. The main reagent is a metallic fluoride to exchange the F atom with the haloalkane.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Mathematics MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Biology MCQs
