This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Preparation of Nitro Compounds”.
1. Which of the following is used as a reactant for the nitration of benzene to form nitrobenzene?
a) HNO2
b) HNO3
c) Mixture of HNO2 AND HNO3
d) Mixture of HNO2 and H2SO4
View Answer
Explanation: Nitration is achieved using a mixture of nitric acid and sulfuric acid, which produce the nitronium ion (NO2+), which the electrophile.
2. What is the name of the following reaction?
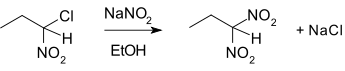
a) Ter meer reaction
b) Riemer tiemann reaction
c) Perkin condensation
d) Ullmann reaction
View Answer
Explanation: In nucleophilic aliphatic substitution, sodium nitrite (NaNO3) replaces an alkyl halide. In the so-called Ter Meer reaction (1876) named after Edmund ter Meer.
3. In Ter Meer reaction, a method of preparation of nitro compound, which of the following used as reagent?
a) HNO2
b) HNO3
c) NaNO2
d) RX (alkyl halide)
View Answer
Explanation: In Ter Meer reaction, which is a nucleophilic aliphatic substitution, sodium nitrite (NaNO2) is used as reactant which replaces an alkyl halide.
4. What will be the product if 1,1-halonitroalkane undergoes nucleophilic aliphatic substitution reaction in presence of ethanol?
a) 1,1-dinitro dimer
b) 1,2-dinitro dimer
c) 1.-nitro compound
d) 2-nitrocompound
View Answer
Explanation: 1,1-dinitro dimer along with sodium chloride will be the products if 1,1-halonitroalkane undergoes nucleophilic aliphatic substitution reaction in presence of ethanol.
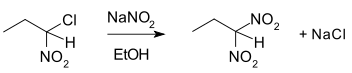
5. What will be the product if 1,1-halonitroalkane undergoes nucleophilic aliphatic substitution reaction in presence of potassiam hydroxide?
a) 1,1-dinitro dimer
b) 1,2-dinitro dimer
c) 1.-nitro compound
d) 2-nitrocompound
View Answer
Explanation: 1,1-dinitro dimer along with sodium chloride will be the products if 1,1-halonitroalkane undergoes nucleophilic aliphatic substitution reaction in presence of potassium hydroxide. Aqueous KOHKOH is alkaline in nature i.e. it dissociates to produce a hydroxide ion. These hydroxide ions act as a strong nucleophile and replace the halogen atom in an alkyl halide.
6. Which of the following is not a natural occurring nitro compound?
a) Chloramphenicol
b) 2-Nitrophenol
c) 3-Nitropropionic acid
d) nitrobenzene
View Answer
Explanation: No information about the natural occurrence of nitrobenzene was found in the readily-available literature. Chloramphenicol is a rare example of a naturally occurring nitro compound. At least some naturally occurring nitro groups arose by the oxidation of amino groups. 2-Nitrophenol is an aggregation pheromone of ticks. 3-Nitropropionic acid found in fungi and plants.
7. Which gives a meta-nitro compound as the main product upon nitration with a nitric acid-sulfuric acid mixture?
a) 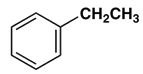
b) 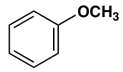
c) 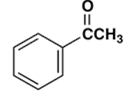
d) 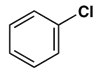
View Answer
Explanation: This activation or deactivation of the benzene ring toward electrophilic substitution may be correlated with the electron donating or electron withdrawing influence of the substituents, as measured by molecular dipole moments. Methoxy group(-OCH3) is an electron donating substituent, which activates the benzene ring toward electrophilic attack and direct substitution to the meta location.
8. Which combination of reagents used in the indicated order with benzene will give m-nitropropylbenzene?
a) i) HNO3/H2SO4, ii) CH3CH2CH2Cl/AlCl3
b) i) CH3CH2CH2Cl/AlCl3, ii) HNO3/H2SO4
c) i) CH3CH2COCl/AlCl3, ii) HNO3/H2SO4, iii) H2NNH2/NaOH
d) i) HNO3/H2SO4, ii) CH3CH2COCl/AlCl3, iii) H2NNH2/NaOH
View Answer
Explanation: Friedel-Crafts reactions are not usually successful with strongly deactivated arenes such as nitrobenzene, and that linear alkyl groups longer than ethyl easily rearrange to secondary alkyls during the Friedel-Crafts alkylation.
9. Nitration of furan using acetyl nitrate also leads to a 2,5-addition product. How can this competing process be suppressed?
a) By using concentrated nitric and sulfuric acids instead of acetyl nitrate
b) By carrying out the reaction at higher temperatures
c) By carrying out the nitration in the presence of pyridine
d) By using nitric acid instead of acetyl nitrate
View Answer
Explanation: 2-Nitrofuran may be nitrated with 70% nitric acid and then affords 2,5-dinitrofuran (67%) and 2,4-dinitrofuran (5%). The acetyl nitrate method usually proceeds by an addition–elimination mechanism and in certain cases the addition products may be isolated and require treatment with a base such as pyridine to effect elimination of acetic acid.
10. Predict the major product of the following reaction.
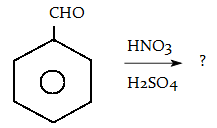
a) o-nitrobenzaldehyde and p-nitrobenzaldehyde
b) m-nitrobenzaldehyde
c) o-formylbenzenesulfonic acid and p-formylbenzenesulfonic acid
d) m-formylbenzenesulfonic acid
View Answer
Explanation: This activation or deactivation of the benzene ring toward electrophilic substitution may be correlated with the electron donating or electron withdrawing influence of the substituents, as measured by molecular dipole moments. CHO- group is a deactivating substituent, and direct substitution to the meta location.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Check Organic Chemistry Books
