This set of Class 12 Chemistry Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Amines Chemical Reactions – 3”.
1. If x and y are the pKb values of p-methylaniline and N,N-dimethylaniline, what is the relation between x and y?
a) x > y
b) x < y
c) x = y
d) x >> y
View Answer
Explanation: The basicity of N-methyl anilines is very slightly higher than that of ring substituted methyl anilines. This is because the methyl group is directly attached to the N atom in N,N-dimethylaniline and the electron effect is more pronounced. But this is only a minute effect.
2. What is the correct order of basic strength of the following compounds?
a) Aniline > Diphenylaniline > Triphenylaniline
b) Aniline > Triphenylaniline > Diphenylaniline
c) Diphenylaniline > Triphenylaniline > Aniline
d) Triphenylaniline > Diphenylaniline > Aniline
View Answer
Explanation: When hydrogen atoms of amino groups are replaced by electron withdrawing groups (in this case, phenyl group), the basic character of the resulting aryl amine decreases.
3. Alkylation of amines with alkyl halides proceeds by _________
a) electrophilic addition
b) electrophilic substitution
c) nucleophilic addition
d) nucleophilic substitution
View Answer
Explanation: The amines itself acts as nucleophiles and attack the alkyl halide at the carbon-halogen bond to substitute the halogen atom by itself. This occurs by production of small amount of acid at each step, which may prevent the reaction by proceeding by protonating the amine.
4. Which of the following is not a likely product of the alkylation of amines with methyl iodide?
a) Primary amine
b) Secondary amine
c) Tertiary amine
d) Quaternary ammonium salt
View Answer
Explanation: Primary and secondary amines react with alkyl halide to form tertiary amines by nucleophilic substitution. When a primary or secondary amine acts as the nucleophile, a secondary or tertiary amine is generated respectively. Finally, the tertiary amine reacts with the remaining methyl iodide to form quaternary ammonium iodide salt.
5. The alkylation of amines with alkyl halides is carried out in the presence of which of the following?
a) Alcohol
b) Acid
c) Base
d) LiAlH4
View Answer
Explanation: Some amount of strong acid is produced at each step of alkylation. This acid can protonate the amine, making the electron lone pair of N unavailable for nucleophilic attack and therefore halt the reaction. For this reason, the acid needs to be neutralised and hence, a base is needed during the reaction.
6. During the acylation of an aliphatic primary amine, which of the following is replaced by an acyl group?
a) NH2 group
b) One H atom of NH2
c) Both H atoms of NH2
d) Alkyl group of the amine
View Answer
Explanation: Aliphatic and aromatic 1° and 2° amines react with acid chlorides, anhydrides and esters by nucleophilic substitution, where the H atom of NH2 or NH group is replaced by the acyl group.
7. Which of the following is formed from the reaction between RNHR’ and R”OCl, where R, R’ and R” are three different alkyl groups?
a) RNR’COR”
b) R2NCOR’
c) R’2NCOR”
d) R”NR’COR
View Answer
Explanation: This an acylation reaction in which the H atom of RNHR’ gets replaced by the COR” group form R”OCl. Thus, the amide formed consists of three different alkyl groups with two attached to the N atom and one attached to the carbonyl carbon.
8. The reaction shown below gives ________
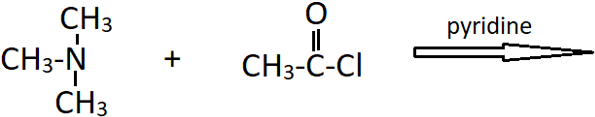
a) N,N-dimethylmethanamide
b) N,N-dimethylethanaminde
c) N-methylethanamide
d) no reaction
View Answer
Explanation: The amine that is treated with CH3COCl is a tertiary amine (N,N-dimethylmethanamine). It does not undergo reaction with acetyl chloride as it does not have a replaceable hydrogen atom. Therefore, for the acylation of an amine, a H atom on nitrogen is required along with the lone pair of electrons.
9. The reaction between ethanamine and acetyl chloride will give N-ethylethanamide only in the presence of a strong base, like pyridine.
a) True
b) False
View Answer
Explanation: A base like pyridine may be used in the acylation of amines only to increase the concentration of the product formed. The base removes the HCl formed during the reaction and shifts the equilibrium to the right-hand side.
10. What are the products formed from the reaction between aniline and ethanoic anhydride?
a) N-Phenylethanamide and hydrochloric acid
b) N-Phenylethanamide and acetic acid
c) N-Methylbenzamide and acetic acid
d) N-Methylbenzamide and hydrochloric acid
View Answer
Explanation: The H atom attached to nitrogen of aniline is replaced by COCH3 group of ethanoic anhydride. This forms N-phenylethanamide (or acetanilide). The by product (acetic acid) is formed by the attachment of the H atom from aniline with remaining CH3COO part of ethanoic anhydride.
11. What is the product of the benzoylation of aniline in the presence of aqueous NaOH?
a) N-Ethylethanamide
b) N-Ethylbenzamide
c) N-Phenylethanamide
d) N-Phenylbenzamide
View Answer
Explanation: Amines react with benzoyl chloride (C6H5COCl) in the presence of a base to form benzoyl derivatives in which the C6H5CO– group is introduced. This is called benzoylation reaction.
12. Which of the following amines does not undergo benzoylation?
a) N-Methylethanamine
b) N-Ethylethanamine
c) N,N-Dimethylethanamine
d) N-Methylbenzenamine
View Answer
Explanation: For benzoylation to take place, there should be a hydrogen atom present at the nitrogen of the amine for the C6H5CO– group to replace. This is not the case in tertiary amines like N,N-dimethylethanamine and hence they do not undergo benzoylation.
13. A certain amine ‘X’ when heated with chloroform and alcoholic KOH, produced no reaction. Identify ‘X’ from the following.
a) Methylamine
b) Ethylamine
c) Ethylmethylamine
d) Aniline
View Answer
Explanation: When an amine is heated with CHCl3 and alc. KOH and does not give any reaction, it indicates that it is a secondary or tertiary amine. This is called as carbylamines test and the compound ‘X’ will be ethylmethylamine, as it is 2° and fails the test.
14. Which of the following compounds is not formed during the heating of ethylamine with chloroform in ethanolic KOH?
a) Propanenitrile
b) Ethyl isocyanide
c) Potassium chloride
d) Water
View Answer
Explanation: Ethylamine on heating with chloroform and ethanolic KOH forms an isocyanide, ethyl isocyanide (CH3CH2NC) along with KCl and H2O. This is known as carbylamines reaction. Propanenitrile (CH3CH2CN) is not formed as it is the cyanide form of ethyl isocyanide.
15. Aniline on carbylamines test gives an unpleasant foul odour.
a) True
b) False
View Answer
Explanation: Aniline is a primary aromatic amine and reacts with chloroform and alc. KOH to give phenyl isocyanide, which is a foul smelling substance.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
