This set of Class 12 Chemistry Chapter 9 Multiple Choice Questions & Answers (MCQs) focuses on “Isomerism in Coordination Compounds – 2”.
1. Which of the following compounds has enantiomers?
a) K3[Fe(CN)6]
b) K3[Al(C2O4)3]
c) K2[Zn(OH)4]
d) K2[PdCl4]
View Answer
Explanation: K3[Fe(CN)6], K2[Zn(OH)4] and K2[PdCl4] are compounds with unidentate ligands and CN of 6 (octahedral), 4 and 4 respectively. Whereas, K3[Al(C2O4)3] is an octahedral compound with a didentate ligand oxalate and shows optical isomerism.
2. How many stereoisomers does the following compound have?
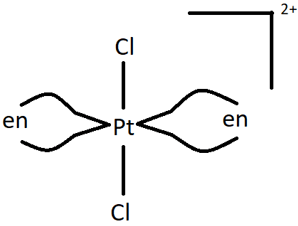
a) 0
b) 2
c) 3
d) 4
View Answer
Explanation: Firstly, the compound can have 2 geometric isomers, cis and trans, depending on whether similar ligands are placed adjacent to or opposite each other. Furthermore, one of them, i.e. the cis form is optically active and has two non-superimposable forms. This means that the cis form is both a geometric isomers as well as an optical isomer. Hence, there are 3 stereoisomers (1 geometric, 1 optical, 1 both geometric and optical).
3. The coordination entity [CrCl2(ox)2]3- is optically active.
a) True
b) False
View Answer
Explanation: [CrCl2(ox)2]3- has two geometric isomers, cis and trans. The trans isomer is achiral and only the cis isomer is optically active. Thus, the statement is not always true.
4. Identify the type of isomerism exhibited by the following structures.
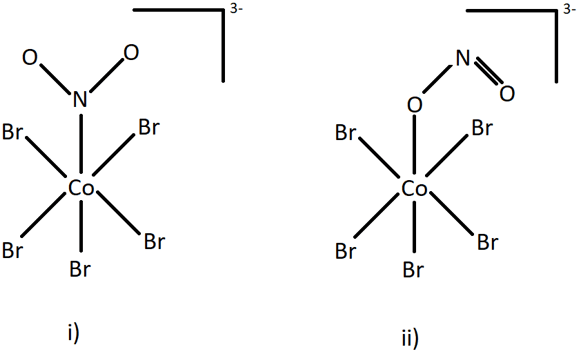
a) Geometric isomerism
b) Optical isomerism
c) Linkage isomerism
d) Coordination isomerism
View Answer
Explanation: Geometric and optical isomerism are types of stereoisomerism in which the chemical bonds remain same. The two structures shown have different chemical bonds between the central atom (Co) and the ligand group (NO2). Nitrate is an ambidentate ligand which can result in two forms in the entities it is present in. If it links through N atom, it forms nitrito-N form (NO2) as in figure i), and if it binds through O atom, it forms nitrito-O form (ONO) as in figure ii). This type of isomerism is called linkage isomerism.
5. Linkage isomerism is seen in compounds having ________ ligand.
a) unidentate
b) polydentate
c) chelate
d) ambidentate
View Answer
Explanation: Ambidentate ligands can bind through either one of their two donor atoms and can result in different forms. These forms are called linkage isomers.
6. Which of the following compounds is not a linkage isomer?
a) Hg[Co(SCN)4]
b) [Cr(H2O)5(NO2)]Cl2
c) [CoCl2(en)2]Cl
d) K[Cr(NH3)2(ONO)4]
View Answer
Explanation: The compounds Hg[Co(SCN)4], [Cr(H2O)5(NO2)]Cl2 and K[Cr(NH3)2(ONO)4] have ambidentate ligands thiocyanate or nitrate in them and these ligands can bind through different donor atoms to produce different structures or isomers.
7. Linkage isomers exhibit different physical properties.
a) True
b) False
View Answer
Explanation: It was observed that [Co(NH3)5(NO2)]Cl2 was either red in colour or yellow depending on whether the nitrite ligand bonded through O atom or N atom respectively, thus showing different physical properties.
8. Which of the following compounds does not have a coordination isomer?
a) [Ag(NH3)2][Ag(CN)2]
b) [Cr(NH3)6][Co(CN)6]
c) [Zn(NH3)4][PtCl4]
d) [Cu(NH3)4][FeCl4]
View Answer
Explanation: Coordination isomerism takes place when exchange of ligands happens between cationic and anionic complex ions having different metal ions. In the compound [Ag(NH3)2][Ag(CN)2], both the complexes have the same metal ion Ag+, and hence does not have a coordination isomer.
9. Identify the coordination isomer of [Fe(CO)4][Zn(CN)4].
a) Tetracyanidozinc(II) tetracarbonylferrate(II)
b) Tetracarbonylzinc(II) tetracyanidoferrate(II)
c) Tetracyanidoiron(II) tetracarbonylzincate(II)
d) Tetracarbonyliron(II) tetracyanidozincate(II)
View Answer
Explanation: The coordination isomer will be [Zn(CO)4][Fe(CN)4] by interchanging the central atoms between the two complexes with [Zn(CO)4]2+ being the cationic complex and [Fe(CN)4]2- being anionic. The naming will be tetracarbonylzinc(II) tetracyanidoferrate(II).
10. The compounds [Co(NH3)5Cl]SO4 and [Co(NH3)5(SO4)]Cl are ________ isomers.
a) linkage
b) coordination
c) ionisation
d) solvate
View Answer
Explanation: The sulphate counter ion is a potential ligand and can displace the Cl atom to make the latter the counter ion.
11. [Co(NH3)5Cl]SO4 + Ag+ = _________
a) AgCl
b) BaSO4
c) white precipitate
d) no reaction
View Answer
Explanation: The compound [Co(NH3)5Cl]SO4 dissociates into a cobalt complex ion and a sulphate ion when dissolved ion water. The sulphate ion does not react with silver ion. On the other hand, the ionisation isomer of [Co(NH3)5Cl]SO4, that is [Co(NH3)5(SO4)]Cl dissociates into cobalt complex ion and Cl ion which reacts with silver ion to precipitate AgCl.
12. The compound [Co(NH3)5(NO2)](NO3)2 does not show _________ isomerism.
a) coordination
b) optical
c) ionisation
d) linkage
View Answer
Explanation: There is only one complex ion and there is no possibility of a coordination isomer. The ionisation isomer is [Co(NH3)5(NO3)](NO3)(NO2) and the linkage isomer is [Co(NH3)5(ONO)](NO3)2.
13. Hydrate isomerism is a form of ________ isomerism.
a) coordination
b) linkage
c) ionisation
d) solvate
View Answer
Explanation: When the solvate involved in solvate isomerism is water molecules; it is called as hydrate isomerism.
14. What is the number of water molecules that are present as ligands in the solvate isomer of hexaaquachromium(III) chloride which is grey-green in colour?
a) 1
b) 4
c) 5
d) 6
View Answer
Explanation: The solvate isomer of [Cr(H2O)6]Cl3 that is grey-green in colour is [Cr(H2O)5Cl]Cl2.H2O. This compound has 5 water molecules directly bonded to metal ion and one water molecule as a free solvent in the crystal lattice.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Mathematics MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Biology MCQs
