This set of Class 12 Chemistry Chapter 15 Multiple Choice Questions & Answers (MCQs) focuses on “Types of Polymerisation Reactions – 1”.
1. Addition polymerisation is also known as _________
a) copolymerisation
b) homopolymerisation
c) step growth polymerisation
d) chain growth polymerisation
View Answer
Explanation: Addition polymerisation takes place through steps leading to the increase in chain length and each step produces reactive intermediates for use in the next stage of the growth of the chain. It may take place between molecules of the same monomer or different monomers.
2. Which of the following cannot undergo addition polymerisation?
a) Ethane
b) Ethene
c) Propylene
d) Vinyl benzene
View Answer
Explanation: The monomers involved in addition polymerisation are unsaturated compounds like alkenes and alkadienes. This is because it does not involve the removal of any molecule and hence requires the breaking of a double bond for it to progress.
3. Which of the following is not a type of addition polymerisation?
a) Free radical polymerisation
b) Polycondensation polymerisation
c) Anionic polymerisation
d) Cationic polymerisation
View Answer
Explanation: Depending on the nature of the reactive species involved, addition polymerisation is classified as free radical, anionic and cationic based on whether the initiator is a free radical, anion or cation respectively.
4. Which of the following is not a suitable initiator for free radical addition polymerisation reaction?
a) Acetyl peroxide
b) Benzoyl peroxide
c) tert-Butyl peroxide
d) Benzoquinone
View Answer
Explanation: Benzoquinone combines with the free radical intermediate to form a highly stable, non-reactive radical because of resonance. This inhibits the further progress of the chain growth and therefore, the reaction stops. Peroxides are very important free radical generating initiators.
5. In free radical mechanism, the step in which two very large free radicals combine with each other is called the _______ step.
a) chain initiating
b) chain propagating
c) chain growth
d) chain terminating
View Answer
Explanation: The final step in which the product radical react with each other to form a polymerised product is called the chain terminating step. The other two steps involve reaction of one free radical with the unsaturated molecule.
6. Identify the chain initiation step of the polymerisation of ethene in the presence of benzoyl peroxide initiator, from the following?
a) C6H5• + C2H4 = C6H5-CH2-CH2•
b) C6H5-CH2-CH2• + C2H4 = C6H5-CH2-CH2-CH2-CH2•
c) C6H5-CH2-CH2-CH2-CH2• = C6H5-(-CH2-CH2-)n-CH2•
d) C6H5-(-CH2-CH2-)n-CH2• + C6H5-(-CH2-CH2-)n-CH2• = C6H5-(-CH2-CH2-)n-C6H5
View Answer
Explanation: Benzoyl peroxide undergoes homolytic fission to from phenyl free radical which acts an initiator. The first step is the addition of this radical to the ethene double bond, thus generating a new and larger free radical. This is called the chain initiating step.
7. Which of the following statements is correct regarding LDP and HDP?
a) Both have different monomers
b) Both have same structures
c) Both have similar preparation conditions
d) Both are chemically inert
View Answer
Explanation: Low-density and high-density polythene have the same monomeric unit, ethene. Both are synthesized under very different pressure and temperatures conditions. LDP has a branches whereas HDP is a linear structure. Both are chemically inert and tough.
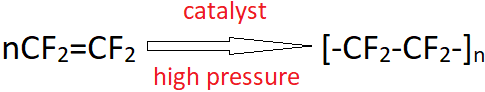
8. Identify the most suitable catalyst for the reaction shown.
a) Dioxygen
b) Ziegler-Natta catalyst
c) Persulphate
d) AlCl3
View Answer
Explanation: This is the addition polymerisation of tetrafluoroethene to give Teflon. It is carried out in the presence of a free radical or persulphate catalyst at high pressures.
9. LDP is used in the making of electrical wires.
a) True
b) False
View Answer
Explanation: LDP is chemically inert and tough but flexible and a poor conductor of electricity. This makes it suitable for use in the insulation of electricity carrying wires.
10. Which of the following is not suitable for the polymerisation of ethene to form high-density polythene?
a) Presence of Ziegler-Natta catalyst
b) Temperature of 500K
c) Pressure of 7 atmospheres
d) Hydrocarbon solvent
View Answer
Explanation: HDP is formed when polymerisation of C2H4 takes place through addition in a hydrocarbon solvent with Ziegler-Natta catalyst at temperature of 333-343K and under a pressure of 6-7 atmospheres. Temperatures and pressure higher than this will favour the formation of LDP.
11. Polymerisation of vinyl cyanide with peroxide catalyst forms _______
a) PVC
b) PAN
c) PET
d) HDP
View Answer
Explanation: Polyacrylonitrile (PAN) is a polymerised product of acrylonitrile (vinyl cyanide) in the presence of a peroxide catalyst. Vinyl cyanide can be prepared by treating ethyne with HCN in the presence of Ba(CN)2 catalyst. PAN is used for making orlon and acrilan.
12. Which of the following is used in non-stick pans?
a) HDP
b) LDP
c) Teflon
d) Orlon
View Answer
Explanation: Teflon (polymer of tetrafluoroethene) is chemically inert and resistant to attacks by corrosive agents. This makes it suitable for use in making oil seals, gaskets and also in non-stick surface coated utensils.
13. What are the monomers of dacron?
a) Ethane-1,2-diol and terephthalic acid
b) Ethylene glycol and phthalic acid
c) 1,3-Butadiene and terephthalic acid
d) Ethylene glycol and 1,3-butadiene
View Answer
Explanation: Dacron or terylene is a condensation polymer formed by the step growth polymerisation of ethylene glycol and terephthalic acid.
14. Terylene is a polyamide.
a) True
b) False
View Answer
Explanation: Terylene is a polycondensation product of a dicarboxylic acid (terephthalic acid) and a diol (ethylene glycol) involving ester (COO) linkages. Hence, it is a polyester.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 12 - Chemistry Books
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
