This set of Class 12 Chemistry Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Carboxylic Acids Chemical Reactions – 3”.
1. Ammonium acetate on removal of ______ forms acetamide.
a) H2
b) NH3
c) OH–
d) H2O
View Answer
Explanation: Ammonium acetate (CH3COO-NH4+) is an ammonium salt which on heating at high temperatures, loses a water molecule to give acetamide (CH3CONH2).
2. Benzoic acid reacts with ______ to give ammonium benzoate salt, which on further dehydration gives benzamide.
a) N2
b) NH3
c) NH4OH
d) HNO3
View Answer
Explanation: Benzoic acid reacts with ammonia in a reversible reaction to form ammonium benzoate by addition reaction. This salt then loses water on heating to form benzamide.
3. The conversion of phthalamide to phthalimide is brought about by the loss of ______ molecule.
a) one H2O
b) two H2O
c) one NH3
d) two NH3
View Answer
Explanation: Phthalic acid on reaction with NH3 forms ammonium phthalate, which on heating loses two H2O molecules, one from each COONH4 group, to form phthalamide. This on further strong heating loses one NH3 molecule to form phthalimide, which is an imide derivative of phthalic anhydride.
4. Which of the following reagents does not reduce the CO group of carboxylic acids to CH2 groups to form alcohols?
a) LiAlH4-ether
b) B2H6-THF
c) H2-CuCr2O4
d) NaBH4
View Answer
Explanation: Carboxylic acids on reduction with LiAlH4, B2H6 or H2 in the presence of suitable medium, are reduced to alcohols. The COOH group is reduced to CH2OH group. Sodium borohydride cannot reduce the carboxyl group.
5. Soda lime consists of NaOH and CaO respectively, in the ratio of _______
a) 1:2
b) 2:1
c) 1:3
d) 3:1
View Answer
Explanation: Soda lime is the 3:1 mixture of sodium hydroxide and calcium oxide, and is used in the decarboxylation reaction (loss of carbon dioxide) of carboxylic acids.
6. Sodium acetate on heating with NaOH and CaO (3:1 mixture) gives _______
a) methane
b) ethane
c) methanol
d) ethanal
View Answer
Explanation: Sodium acetate undergoes decarboxylation (loss of CO2) when heated with soda lime. This is proceeded by the loss of COONa from the sodium salt and NaO from sodium hydroxide. They combine to form CH4 and Na2CO3. The hydrocarbon formed has one less carbon atom than the parent acid.
7. Which of the following acids on Kolbe’s electrolysis gives ethane?
a) HCOOK
b) HCOONa
c) CH3COOK
d) CH3CH2COONa
View Answer
Explanation: Aqueous solutions of sodium or potassium salts of carboxylic acids on electrolysis, undergo decarboxylation and form hydrocarbons having twice the number of carbon atoms as present in the alkyl group of the acid. Since ethane has two C atoms, the alkyl group of the acid should be CH3.
8. Identify X in the following reaction.
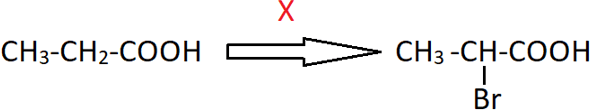
a) i) Br2-red P; ii) H2O
b) i) Cl2-red P; ii) H2O
c) PBr3-H2O
d) PBr5-H2O
View Answer
Explanation: Propanoic acid undergoes bromination at alpha-carbon in the presence of red phosphorus to give α-Bromopropanoic acid. This is known as Hell-Volhard-Zelinsky reaction.
9. Methanoic acid undergoes Hell-Volhard-Zelinsky reaction.
a) True
b) False
View Answer
Explanation: Methanoic acid (HCOOH) consists of only one carbon atom and therefore does not have an alpha-hydrogen atom that can be replaced by a halogen atom.
10. What is formed when benzoic acid undergoes nitration in the presence of conc. HNO3 and conc. H2SO4?
a) o-Nitrobenzoic acid
b) m-Nitrobenzoic acid
c) p-Nitrobenzoic acid
d) 3,5-Dinitrobenzoic acid
View Answer
Explanation: Benzoic acid undergoes ring substitution in which the COOH group acts as a deactivating group and is meta-directing in nature.
11. Benzoic acid undergoes Friedel-Crafts alkylation to form o- and p-Toluic acid.
a) True
b) False
View Answer
Explanation: The COOH group is a deactivating group and does not undergo Friedel-Crafts alkylation. The catalyst AlCl3 being a Lewis acid, gets bonded to the carboxyl group, and the methyl group does not get substituted in the ring.
12. Benzoic acid reacts with ______ to give m-Bromobenzoic acid.
a) Br2/light
b) Br2-FeBr3
c) NaBr
d) PBr3/heat
View Answer
Explanation: Benzoic acid undergoes electrophilic substitution with bromine at the meta position in the presence of FeBr3 to form 3-bromobenzoic acid and hydrogen bromide as side product.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
