This set of Class 12 Chemistry Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Aldehydes, Ketones, and Carboxylic Acids”. These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation.
1. Which of the following methods cannot produce aldehydes?
a) Oxidation of primary alcohols
b) Dehydrogenation of secondary alcohols
c) Ozonolysis of alkenes
d) Hydration of ethyne with acid
View Answer
Explanation: The dehydrogenation of secondary alcohols give ketones. Aldehydes are obtained by the dehydrogenation of primary alcohols.
2. Which of the following reactions can produce ketones?
a) Oxidation of primary alcohols
b) Dehydrogenation of primary alcohols
c) Dehydrogenation of tertiary alcohols
d) Oxidation of secondary alcohols
View Answer
Explanation: Oxidation and dehydrogenation of secondary alcohols results in ketones. The same reactions with primary alcohols give aldehydes.
3. Conversion of propyne to acetone requires three important reagents. Identify which of the following is not one of the three?
a) Water
b) Zinc dust
c) H2SO4
d) HgSO4
View Answer
Explanation: Addition of water to propyne in the presence of H2SO4 and HgSO4 gives acetone. Zinc dust is an important reagent in the ozonolysis of alkenes.
4. What is the catalyst used in the hydrogenation of acetyl chloride to produce ethanal?
a) Pt over BaSO4
b) Pt over CuSO4
c) Pd over BaSO4
d) Pd over CuSO4
View Answer
Explanation: Acyl chlorides are hydrogenated in the presence of catalyst palladium over barium sulphate. This is known as Rosenmund reaction.
5. Which of the following carbonyl compounds can be prepared from Rosenmund reaction?
a) Methanal
b) Acetone
c) Butanone
d) Benzaldehyde
View Answer
Explanation: Rosenmund reaction is exclusively used for the preparation of aldehydes by the substitution of chloride by hydrogen. Given this, methanal cannot be formed from this reaction because its corresponding acyl chloride, i.e., formyl chloride, is unstable at room temperature. Benzaldehyde is formed from benzoyl chloride.
6. Which of the following is required in Stephen reaction?
a) LiCl
b) NiCl2
c) SnCl2
d) TiCl4
View Answer
Explanation: Nitriles are converted to respective imines with SnCl2 in the presence of HCl, which on hydrolysis gives corresponding aldehyde.
7. Which of the following compounds helps in reducing esters to aldehydes?
a) BINAL-H
b) DIBAL-H
c) DIPT
d) TBAF
View Answer
Explanation: DIBAL-H or diisobutylaluminium hydride is a reducing agent which are used to reduce nitriles to imines or esters to aldehydes. These are important in the preparation of aldehydes.
8. Identify ‘X’ in the reaction given below.
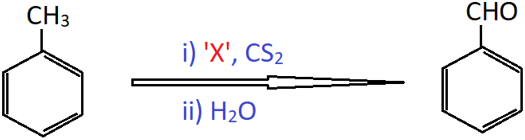
a) CrO3
b) CrO2Cl2
c) Alkaline KMnO4
d) Anhydrous AlCl3
View Answer
Explanation: This is known as Etard reaction. The chromyl chloride oxidises the methyl group to a chromium complex in CS2. This complex on hydrolysis gives benzaldehyde.
9. Benzaldehyde can be obtained from the hydrolysis of the product formed during side chain chlorination of toluene.
a) True
b) False
View Answer
Explanation: Side chain chlorination of toluene in the presence of light (represented by hv) gives benzal chloride which on hydrolysis gives benzaldehyde. This is an important commercial method for the production of benzaldehyde.
10. Identify the reagent(s) for the conversion of chlorobenzene to 3-chlorobenzaldehyde.
a) CrO3 and (CH3CO)2O
b) CrO2Cl2; H2O
c) Cl2/hv; H2O
d) CO, HCl and CuCl
View Answer
Explanation: When benzene or its derivative is treated with CO and HCl in the presence of anhydrous AlCl3 or CuCl, it gives a benzaldehyde or substituted benzaldehyde. This is known as Gatterman-Koch reaction.
11. Acetyl chloride reacts with _______ to give butan-2-one.
a) cadmium chloride
b) methyl magnesium chloride
c) dimethyl cadmium
d) diethyl cadmium
View Answer
Explanation: For butan-2-one to form from acetyl chloride, the Cl group needs to be replaced by an ethyl group. This ethyl group is obtained from diethyl cadmium which is produced from the reaction between ethyl magnesium bromide and cadmium chloride.
12. What is formed after the hydrolysis of the product of the reaction between benzonitrile and methyl magnesium bromide in dry ether?
a) Phenyl acetaldehyde
b) Acetophenone
c) 1-Phenylpropanone
d) Benzaldehyde
View Answer
Explanation: When a nitrile is treated with a Grignard reagent in the presence of dry ether, an addition product is formed which on hydrolysis gives a ketone.
13. Friedel-Crafts benzoylation of benzene gives ________
a) acetophenone
b) propiophenone
c) benzophenone
d) no reaction
View Answer
Explanation: When benzene is treated with benzoyl chloride in the presence of anhydrous aluminium chloride, it forms benzophenone which is an aromatic ketone.
14. Identify the reagent for the conversion of but-2-ene to ethanal.
a) O3/H2O-Zn dust
b) H2O, H2SO4, HgSO4
c) PCC
d) DIBAL-H
View Answer
Explanation: But-2-ene on ozonolysis followed by reaction with water and zinc dust gives ethanal or sometimes a mixture of an aldehyde and ketone depending on the substitution pattern.
15. Which of the following reactions does not give benzaldehyde?
a) Rosenmund reaction
b) Etard reaction
c) Gatterman-Koch reaction
d) Friedel-Craft acylation reaction
View Answer
Explanation: Friedel-Crafts acylation of benzene produces an aromatic ketone and not benzaldehyde. Benzene on Gatterman-Koch reaction gives benzaldehyde, so does toluene on Etard reaction. Rosenmund reaction also gives benzaldehyde but from benzoyl chloride.
More MCQs on Class 12 Chemistry Chapter 12:
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 2)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 3)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 4)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 5)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 6)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 7)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 8)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 9)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 10)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 11)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 12)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 13)
- Chapter 12 – Aldehydes, Ketones, and Carboxylic Acids MCQ (Set 14)
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Physics MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Biology MCQs
- Check Class 12 - Chemistry Books
