This set of Class 12 Chemistry Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Carboxylic Acids Chemical Reactions – 1”.
1. The reaction of carboxylic acids with NaHCO3 produces ______ which helps it to differentiate it from phenols.
a) H2O
b) CO
c) CO2
d) NaCl
View Answer
Explanation: Unlike phenols, carboxylic acids react with weak bases like hydrogen carbonates to give carbon dioxide, along with sodium carboxylate salt and water.
2. The pKa value is equivalent to ________
a) logKa
b) -logKa
c) logKeq
d) -logKeq
View Answer
Explanation: Keq is the equilibrium constant of a reaction and Ka is the acid dissociation constant which is Keq[H2O]. The strength of an acid is generally by its pKa value rather than its Ka value.
3. If the pKa value of acetic acid is 4.76, predict the pKa value of HCl.
a) -7
b) 4.2
c) 7
d) 10
View Answer
Explanation: Generally, carboxylic acids are weaker acids than mineral acids like hydrochloric acid. Also, mineral acids are strong with pKa values usually less than 1. Hence, the pKa value of carboxylic acids will be higher.
4. What is the relation between the acidic strength of A and B?
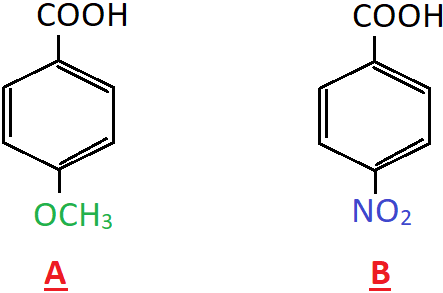
a) A = B
b) A > B
c) A < B
d) A >> B
View Answer
Explanation: Compound A has a methoxy group (electron donating) and compound B has nitro group (electron withdrawing). In simple terms, the electron withdrawing groups stabilise the carboxylate ion and strengthen the acid, whereas electron donation groups weaken the acid.
5. Which of the following is the stronger acid?
a) Acetic acid
b) Propanoic acid
c) Isobutyric acid
d) 2,2-Dimethylpropanoic acid
View Answer
Explanation: As the size of alkyl group (electron releasing) increases, the electron density on the O of OH group increases, and makes release of H+ ion more difficult. Thus, the release of proton from acetic acid will be easier compared to the higher acids, and is therefore the stronger acid.
6. Which of the following is the strongest acid?
a) CH3COOH
b) CH2ClCOOH
c) CHCl2COOH
d) CCl3COOH
View Answer
Explanation: The -I inductive effect increases as the number of Cl atoms (electron withdrawing substituents) increases. The carboxylate ion becomes more stable as more negative charge is dispersed, thereby strengthening the acid.
7. Which of the following has the highest pKa value?
a) Bromoacetic acid
b) Chloroacetic acid
c) Fluoroacetic acid
d) Iodoacetic acid
View Answer
Explanation: Higher the electronegativity of a halogen, greater will be its electron withdrawing effect, and stronger will be the acid. Since electronegativity of halogens decrease in the order F > Cl > Br > I, iodoacetic acid will be the weakest and hence will have the highest pKa value.
8. Which of the following will have the highest acidic strength?
a) Butanoic acid
b) 2-Chlorobutanoic acid
c) 3-Chlorobutanoic acid
d) 4-Chlorobutanoic acid
View Answer
Explanation: Halogen substituted carboxylic acids are stronger acid than their respective parent acids, because of the electron withdrawing and stabilizing nature of the halogen. Furthermore, as the distance of the halogen form the COOH group increases, the electron withdrawing influence decreases, and therefore the acidic character decreases.
9. What is the correct order of acidity of the following?
a) Acetic acid > Acrylic acid > Propiolic acid
b) Acetic acid > Propiolic acid > Acrylic acid
c) Propiolic acid > Acrylic acid > Acetic acid
d) Acrylic acid > Propiolic acid > Acetic acid
View Answer
Explanation: The electronegativity of carbon increases from sp3 to sp2 to sp hybridisation. The carbon attached to the carbonyl carbon of propionic acid id sp hybridised (due to triple bond) ang has a higher inductive effect on the COOH group and as a result has higher acidity compared to acrylic acid (sp2 carbon) or acetic acid (sp3 carbon).
10. It is given that the following compound has a higher pKa value than benzoic acid. Which is the most probable substituent group X of the compound?
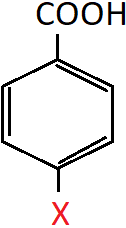
a) OH
b) Cl
c) CN
d) NO2
View Answer
Explanation: Given that the compound has a higher pKa value than benzoic acid, it should have weaker acidic strength than benzoic acid. This means, that X should be an electron donating group that destabilises the compound and reduces its acidic strength. Since is an electron releasing group, the X group should be OH.
11. Acrylic acid is a stronger acid than benzoic acid.
a) True
b) False
View Answer
Explanation: Both compounds contain a sp2 hybridised carbon atom next to the COOH group, but the double bond of a benzene ring is less electron releasing in nature than the vinyl carbon double bond of acrylic acid. This is because delocalisation in the resonance structures of benzoic acid destroys its aromatic character. Hence, benzoic acid is stronger than acrylic acid.
12. What is the correct order of pKa values of the following acids?
a) Benzoic acid > m-Nitrobenzoic acid > p-Nitrobenzoic acid
b) Benzoic acid > p-Nitrobenzoic acid > m-Nitrobenzoic acid
c) p-Nitrobenzoic acid > m-Nitrobenzoic acid > Benzoic acid
d) m-Nitrobenzoic acid > p-Nitrobenzoic acid > Benzoic acid
View Answer
Explanation: Nitro group is electron withdrawing in nature and increases the acidic strength of acids. The electron withdrawing effect of NO2 is pronounced at para position more than meta position, hence the p-isomer is more acidic and has a lower pKa value than m-isomer.
13. Which of the following is the most acidic?
a) Benzoic acid
b) o-Toluic acid
c) m-Toluic acid
d) p-Toluic acid
View Answer
Explanation: Although CH3 is an electron releasing group and should reduce the acidic character of acids by destabilizing it, the presence of CH3 group at ortho position has a reverse effect and increases the acidic strength compared to benzoic acid. This is due to a combination of stearic and electronic factors.
14. Which of the following has a higher acidic character than benzoic acid?
a) Acetic acid
b) p-Methoxybenzoic acid
c) p-Bromobenzoic acid
d) p-Aminobenzoic acid
View Answer
Explanation: Bromine is an electron withdrawing group that disperse the negative charge on the carboxylate ion and strengthens its acidic character. p-Bromobenzoic acid has a pKa value of 3.96 compared to 4.19, which is the pKa value of benzoic acid.
15. The presence of CF3 group in an acid gives a higher acidic strength compared to the presence of NO2.
a) True
b) False
View Answer
Explanation: CF3 group is highly electronegative due to the presence of three F atoms and has a greater electron withdrawing and stabilizing influence on carboxylate ion than NO2 group.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Mathematics MCQs
- Check Class 12 - Chemistry Books
- Practice Class 12 - Physics MCQs
