This set of Tricky Organic Chemistry Questions and Answers focuses on “Electrophilic Aromatic Substitution”.
1. What will be the product formed when phenol reacts with Br2 in CCl4 medium?
a) 3-Bromophenol
b) 4- Bromophenol
c) 3,5-Dibromophenol
d) 2,4,6-Tribromophenol
View Answer
Explanation: Because the non-polar solvent (i.e. CCl4) induces a weaker dipole in Br-Br as compared to the polar solvent (i.e. water), a weaker electrophile (Br+) is produced that undergoes electrophilic aromatic substitution with the benzene ring. Consequently, we get mono-bromination with Br (in CCl4) with compared with tri-bromination Br (in aq.).
2. What is the electrophile in the electrophilic substitution reaction of benzene using oleum and conc. H2SO4?
a) SO3
b) NO3
c) NO2+
d) NO+
View Answer
Explanation: This reaction is sulphonation of benzene ring in which SO3 is the real attacking electrophile and oleum will increase the concentration of SO3.
3. What will be the attacking electrophile in this reaction?
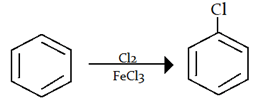
a) Carbanion
b) Halonium
c) Radical
d) Transition state
View Answer
Explanation: In halogenation of benzene ring halonium ion (Cl+) will attack at benzene ring.
4. Which of the following aromatic compounds undergo Friedel–Crafts alkylation with methyl chloride and aluminum chloride?
a) Benzoic acid
b) Nitrobenzene
c) Toluene
d) Aniline
View Answer
Explanation: Toluene will undergo Friedel–Crafts alkylation with methyl chloride and aluminum chloride as because as weak electron withdrawing functional group — will not make the ring less electron dense, hence more reactive in an aromatic electrophilic substitution as compared to electron withdrawing group (benzoic acid, nitrobenzene, aniline).
5. What will be the product in the given reaction?
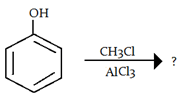
a) m-chlorophenol
b) o-chlorophenol and p-chlorophenol
c) o-hydroxytoluene and p-hydroxytoluene
d) m-hydroxytoluene
View Answer
Explanation: -OH is a ortho and para directing group so attack of electrophile (carbocation- CH3) will attack there, as shown below:
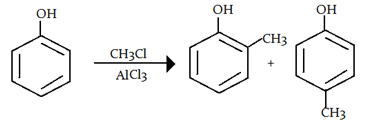
6. What is the electrophile in the acylation of benzene?
a) AlCl3
b) CO+
c) Cl+
d) R-CO+
View Answer
Explanation: The electrophile in the electrophilic substitution reaction of acetyl chloride (CH3COCl) and AlCl3 reacting with benzene is R-CO+.
7. How would you synthesis the following reaction?
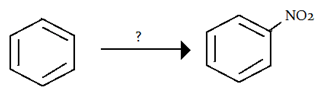
a) conc. HNO3 + conc. H2SO4
b) conc. HNO3
c) anhydrous AlCl3 + Ph-NO2
d) conc. H2SO4 + Oleum
View Answer
Explanation: This reaction is nitration of benzene and conc. HNO3 and conc. H2SO4 is used to carry out this synthesis although HNO2 + HNO3 can also be used.
8. Which of the following reactants can be used to carry out following reaction?
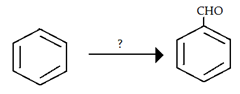
a) conc. HNO3 + conc. H2SO4
b) HCl + CO + AlCl3
c) anhydrous AlCl3 + Ph-NO2
d) conc. H2SO4 + Oleum
View Answer
Explanation: This is Gattermann Koch Reaction in which HCl + CO + AlCl3 are used as reactant. H-CO+ is the real attacking electrophile.
9. What will be the product in the given reaction?
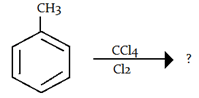
a) m-chlorotoluene
b) o-chlorotoluene and p-chlorotoluene
c) 1-chloro-3-methylbenzene
d) no reaction
View Answer
Explanation: Here no reaction will take place because CCl4 + Cl2 show addition reaction and benzene don’t give addition reaction.
10. Which of the following is the most activating in electrophilic aromatic substitution?
a) -NO2
b) -NHCOCH3
c) -CN
d) -NH2
View Answer
Explanation: Groups with unshared pairs of electrons, such as the amino group of aniline, are strongly activating and ortho/para-directing by resonance. Such activating groups donate those unshared electrons to the pi system, creating a negative charge on the ortho and para positions. These positions are thus the most reactive towards an electron-poor electrophile. The highest electron density is located on both ortho and para positions, although this increased reactivity might be offset by steric hindrance between substituent and electrophile.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice tricky questions and answers on all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
