This set of Class 12 Chemistry Chapter 9 Multiple Choice Questions & Answers (MCQs) focuses on “Coordination Compounds”. These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation.
1. Which of the following is the coordination entity in K2[Zn(OH)4]?
a) K+
b) Zn2+
c) OH–
d) [Zn(OH)4]2-
View Answer
Explanation: A coordination entity consists of a central metal atom bonded to a fixed number of atoms/molecules. Here, K+ is the counter ion, OH is the molecule bonded to the central atom which is Zn.
2. The central atom/ion of a coordination complex is also referred to as ________
a) Lewis acid
b) Lewis base
c) Bronsted-Lowry acid
d) Bronsted-Lowry base
View Answer
Explanation: A Lewis acid is a species that can accept an electron pair. All cations are Lewis acids. Since the central atom of a coordination complex is metal and always accept electrons, it is a Lewis acid.
3. Identify the Lewis acid in K3[Al(C2O4)3].
a) K+
b) Al
c) Al3+
d) [Al(C2O4)3]3-
View Answer
Explanation: Aluminium ion is the Lewis acid as it can accept 3 electrons from the donor atom to form the complex [Al(C2O4)3]3-.
4. Which of the following is the central atom/ion in [CoCl(NH3)5]2+?
a) Co
b) Co2+
c) Co3+
d) Cl–
View Answer
Explanation: The central ion in the given complex ion is cobalt as it accepts electrons to form a bond with Cl atom and ammonia molecules. Since the primary valence of Co in this compound is +3, the ion in Co3+.
5. Which of the following cannot be a ligand?
a) Ni2+
b) Cl–
c) H2O
d) NH3
View Answer
Explanation: The ions/molecules bound to the central atom/ion is called a ligand. Ni2+ is a metal ion, and according to Werner the secondary valences can be satisfied only by neutral molecules or negative ions. Cl–, H2O and NH3 are all possible ligands.
6. How many donor atoms can EDTA4- ligand bind through?
a) 2
b) 4
c) 6
d) 8
View Answer
Explanation: EDTA4- is a hexadentate ligand which can bind through two nitrogen and four oxygen atoms to a central metal ion, giving it a total of 2 + 4 = 6 donor atoms.
7. A protein can exist in a coordination entity as a ligand.
a) True
b) False
View Answer
Explanation: A protein is a macromolecule that consists of atoms that may donate electrons to a metal ion to form a coordinate bond, and hence act as a ligand.
8. Which is the donor atom in the coordinate bond shown below?
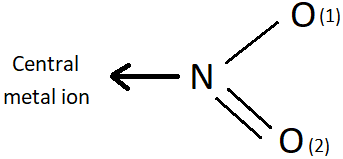
a) N
b) O1
c) O2
d) Both N and O
View Answer
Explanation: This is nitrito-N, formed by ambidentate ligand NO2- ion when it bonds through its N atom to the central metal ion. The other form is nitrito-O, which is formed when NO2- coordinates through the O1 atom.
9. The SCN– ion is a unidentate ligand.
a) True
b) False
View Answer
Explanation: SCN– is an ambidentate ligand that can either bond through S atom or N atom to a central atom/ion. But, at a time there is only a single donor atom, either S or N, making it a unidentate ligand. For a fact, all ambidentate ligands are unidentate.
10. Coordination number is a characteristic of which of the following?
a) Central atom
b) Ligand
c) Coordination entity
d) Coordination compound
View Answer
Explanation: Coordination number is also known as the secondary valence of a central metal ion in a complex and is defined as the number of donor atoms it is directly bonded to. Hence, coordination number is a quantity associated with the metal ion.
11. What is the coordination number of chromium in K3[Cr(C2O4)3]?
a) 1
b) 2
c) 3
d) 6
View Answer
Explanation: There are three oxalate groups attached to the central ion in the given compound, but since oxalate is a didentate ligand, there are a total of 6 donor atoms to which the metal is directly bonded. Thus, the CN of Cr in K3[Cr(C2O4)3] is 6.
12. Identify the coordination sphere in the compound K4[Fe(CN)6].
a) K+
b) Fe2+
c) [Fe(CN)6]4-
d) CN–
View Answer
Explanation: The coordination sphere refers to the square bracket within which the central atom/ion and the groups attached to it are enclosed. This is a non-ionisable group as compared to the entity outside the bracket, which is ionisable and is known as the counter ion.
More MCQs on Class 12 Chemistry Chapter 9:
- Chapter 9 – Coordination Compounds MCQ (Set 2)
- Chapter 9 – Coordination Compounds MCQ (Set 3)
- Chapter 9 – Coordination Compounds MCQ (Set 4)
- Chapter 9 – Coordination Compounds MCQ (Set 5)
- Chapter 9 – Coordination Compounds MCQ (Set 6)
- Chapter 9 – Coordination Compounds MCQ (Set 7)
- Chapter 9 – Coordination Compounds MCQ (Set 8)
- Chapter 9 – Coordination Compounds MCQ (Set 9)
- Chapter 9 – Coordination Compounds MCQ (Set 10)
- Chapter 9 – Coordination Compounds MCQ (Set 11)
- Chapter 9 – Coordination Compounds MCQ (Set 12)
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
- Check Class 12 - Chemistry Books
