This set of Class 12 Chemistry Chapter 9 Multiple Choice Questions & Answers (MCQs) focuses on “Nomenclature of Coordination Compounds – 2”.
1. Identify the correct naming for K3[Fe(CN)6].
a) Tripotassium hexacyanidoferrate(III)
b) Potassium hexacyanoferrate(III)
c) Tripotassium hexacyanoferrate(III)
d) Potassium hexacyanidoferrate(III)
View Answer
Explanation: The number of cations or anions is not denoted in the name of a coordination compound. In this case, the counter ion is named as potassium and not tripotassium. Also, CN is an anionic ligand and should end with -o, with the correct name being cyanido and not cyano.
2. Identify the correct naming for [Zn(OH)4]2-.
a) Zinc tetrahydroxide
b) Tetrahydroxozincate(II)
c) Tetrahydroxidozincate(II)
d) Tetrahydroxylzincate(II)
View Answer
Explanation: The ligand OH is a negative group and should end with -o. The correct name for the OH group is hydroxide and the metal is mentioned after the ligands.
3. Identify the correct naming for [Co(ONO)(NH3)5]2+.
a) Pentaamminenitritocolbalt(III)
b) Pentaamminenitrito-O-cobalt(III)
c) Pentaamminenitrito-N-cobalt(III)
d) Pentaamminenitrosylcobalt(III)
View Answer
Explanation: Nitrate is an ambidentate ligand and can bind through either O or N atom. In this case, it is written as (ONO) which implies that it binds through O atom, hence giving it the name nitrito-O.
4. Identify the correct formula for hexaaquamanganese(II) ion.
a) [Mn(H2O)6]2+
b) [Mn(H2O)6]2-
c) [Mn2(H2O)6]+
d) [Mn(H2O)6]+2
View Answer
Explanation: The parenthesis after manganese represents its oxidation state (+2) which is indicated outside the square bracket with sign following the number. The H2O molecule is a neutral ligand named as aqua.
5. Identify the correct formula for potassium tetracyanonickelate(II).
a) K[Ni(CN)4]
b) K[Ni(CN)4]2
c) K2[Ni(CN)4]
d) K2[Ni(CN)4]2
View Answer
Explanation: CN is anionic with charge -1 and there are 4 of them. Ni is given to have oxidation number +2 in the name of the compound. So, the total charge on the compound will be -4 + 2 = -2. This must be balanced by potassium ions which have charge of +1, so two of them will be required. Hence, the formula is K2[Ni(CN)4].
6. The term inside the parenthesis in the formula of mercury(I) tetrathiocyanato-S-cobaltate(III) is _________
a) Hg
b) SCN
c) NCS
d) Co
View Answer
Explanation: When the ligands are polyatomic, its formula is enclosed in parenthesis. The formula for the given compound is Hg[Co(SCN)4], where the thiocyanate ligand binds through the S atom.
7. Which of the following representation of the complex ion is correct according to IUPAC?
a) [PtBrCl(NH3)(NO2)]–
b) [PtBrCl(NO2)(NH3)]–
c) [Pt(NH3)BrCl(NO2)]–
d) [Pt(NO2)BrCl(NH3)]–
View Answer
Explanation: The ligands are bromine, chlorine, ammine and nitrite and according to IUPAC nomenclature they should be listed as per alphabetical order which is ammine (NH3), bromine (Br), chlorine (Cl) and nitrite (NO2).
8. An imaginary coordination entity named dipalidoqalidoralidometal(IV) according to the IUPAC norms, consists of ligands palide(P), qalide(Q) and ralide(R), all having charge of magnitude 1. The central atom is metal (M). Which of the following options best suits the complex described?
a) 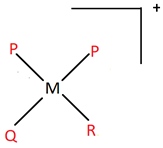
b) 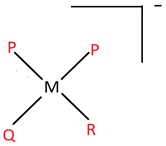
c) 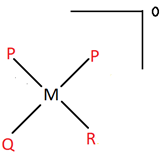
d) 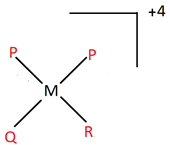
View Answer
Explanation: All the ligands end with -o, which implies they are anionic and have charge -1. The oxidation number of the central atom is given as +4. There are a total of 4 ligands (2 P, 1 Q and 1 R) in the entity. Hence, the overall charge on the complex is +4 – 4 = 0.
9. Which of the following is not enclosed in parenthesis?
a) Polyatomic ligands in the formula of coordination compounds
b) Ligand abbreviations in the formula of coordination compounds
c) Oxidation number of central metal in the naming of coordination compounds
d) The coordination entity in the formula of coordination compounds
View Answer
Explanation: The coordination entity consisting of the metal atom/ion followed by ligands is enclosed within square brackets and not in parenthesis.
10. The prefixes bis, tris and tetrakis are used to indicate the number of individual ligands in the coordination entity.
a) True
b) False
View Answer
Explanation: Mono, di, tri, etc. are used when name of ligands don’t have a numerical prefix. If not, then bis, tris and tetrakis are used as prefixes.
Sanfoundry Global Education & Learning Series – Chemistry – Class 12.
To practice all chapters and topics of class 12 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Mathematics MCQs
- Practice Class 12 - Biology MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
- Check Class 12 - Chemistry Books
