This set of Organic Chemistry written test Questions & Answers focuses on “Chemical Properties of Carboxylic Acids”.
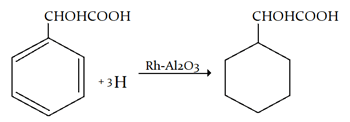
1. Hydrogenation of C6H5CHOH−COOH over Rh−Al2O3 catalyst in methanol gives which of the following?
a) C6H5CH2COOH
b) C6H11CHOHCOOH
c) C6H5CHOHCH2OH
d) C6H11CH2COOH
View Answer
Explanation: Hydrogenation of C6H5CHOH−COOH over Rh−Al2O3 catalyst in methanol gives C6H11CHOHCOOH.
2. In the anion HCOO− the two carbon-oxygen bonds are found to be of equal length. What is the reason for it?
a) Electronic orbitals of carbon atom are hybridised
b) The C = O bond is weaker than the C – O bond
c) The anion HCOO− has two resonating structures
d) The anion is obtained by removal of a proton form the acid molecule
View Answer
Explanation: Anion HCOO− has two resonating structure (identical).
3. In a set of the given reactions, acetic acid yielded a product C
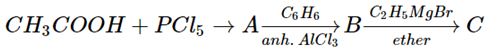
What would be product C?
a) CH3−C-C2H5(OH)C6H5
b) CH3CH(OH)C2H5
c) CH3COC6H5
d) CH3CH(OH)C6H5
View Answer
Explanation: In a set of the given reactions, acetic acid yielded a product C is CH3−C-C2H5(OH)C6H5.
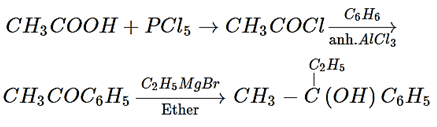
4. Carboxylic acids are more acidic than phenol and alcohol because of which of the following?
a) Intermolecular hydrogen bonding
b) Formation of dimers
c) Highly acidic hydrogen
d) Resonance stabilization of their conjugate base
View Answer
Explanation: Resonance stabilization of their conjugate base i.e., carboxylate ion.
5. When propionic acid is treated with aqueous sodium bicarbonate CO2 is liberated. The ‘C’ of CO2 comes from which of the following?
a) Methyl group
b) Carboxylic acid group
c) Methylene group
d) Bicarbonate
View Answer
Explanation: When propionic acid is treated with aqueous sodium bicarbonate CO2 is liberated and ‘C’ of CO2 comes from bicarbonate.
CH3CH2COOH(aq) + NahCO3(aq) → CH3CH2COONa + CO2 + H2O.
propionic acid sod.bicarbonate
6. The compound not soluble in acetic acid is which of the following?
a) CaCO3
b) CaO
c) CaC2O4
d) Ca(OH)2
View Answer
Explanation: CaC2O4 is a salt of oxalic acid which is more acidic than acetic acid, so it is insoluble in acetic acid.
7. Identify the wrong statement from the following?
a) Salicylic acid’s a monobasic acid
b) Methyl salicylate is an ester
c) Salicylic acid gives violet colour with neutral ferric chloride as well as brisk effervescence with sodium bicarbonate
d) Methyl salicylate does not occur in natural oils
View Answer
Explanation: Methyl salicylate occurs in natural essential oils like winter green.
8. When CH3COOH reacts with CH3−Mg−X, which of the following is formed?
a) CH3COX is formed
b) Hydrocarbon is formed
c) Acetone is formed
d) Alcohol is formed
View Answer
Explanation: When CH3COOH reacts with CH3−Mg−X formed as hydrocarbon.
CH3COOH + CH3 – Mg – X → CH3 – CH3
9. Hydrolysis of an ester gives a carboxylic acid which on Kolbe’s electrolysis yields ethane. The ester is which of the following?
a) Ethyl methonoate
b) Methyl ethanoate
c) Propylamine
d) Ethylamine
View Answer
Explanation: Hydrolysis of an ester gives a carboxylic acid which on Kolbe’s electrolysis yields ethane, this ester is methyl ethanoate.
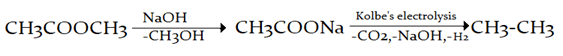
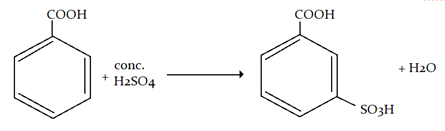
10. Sulphonation of benzoic acid produces mainly which of the following?
a) o-sulphobenzoic acid
b) m-sulphobenzoic acid
c) p-sulphobenzoic acid
d) o- and p-sulphobenzoic acid
View Answer
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all written questions on Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship