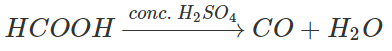This set of Organic Chemistry Objective Questions & Answers focuses on “Physical Properties of Carboxylic Acids”.
1. Which of the following acids has the smallest dissociation constant?
a) CH3CHFCOOH
b) FCH2CH2COOH
c) BrCH2CH2COOH
d) CH3CHBrCOOH
View Answer
Explanation: BrCH2CH2COOH is least acidic or has less Ka i.e., dissociation constant. It is A due to lesser -I effect of Br than F and B Br atom further away form −COOH group.
2. The vapour of a carboxylic acid HA when passed over MnO2 at 573 K yields propanone. What is the acid HA?
a) Methanoic acid
b) Ethanoic acid
c) Propanoic acid
d) Butanoic acid
View Answer
Explanation: The vapour of ethanoic acid (HA) when passed over MnO2 at 573 K yields propanone.
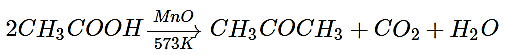
3. Which acid is strongest or Which is most acidic?
a) Cl2CH.COOH
b) ClCH2COOH
c) CH3COOH
d) Cl3C.COOH
View Answer
Explanation: Presence of -I effect chlorine atom increases the acidic nature by withdrawing electrons.
Cl3CCOOH > Cl2CHCOOH > Cl−CH2−COOH > CH3COOH
4. The acid which reduces Fehling solution is which of the following?
a) Methanoic acid
b) Ethanoic acid
c) Butanoic acid
d) Propanoic acid
View Answer
Explanation: Methanoic acid resemble with aldehyde due to its structure. So, it reduces Fehling reagent.
5. Which class of compounds shows H-bonding even more than in alcohols?
a) Phenols
b) Carboxylic acids
c) Ethers
d) Aldehydes
View Answer
Explanation: Forms H-bonding by means two highly electronegative atoms present in it.
6. Which of the following is the strongest acid?
a) CH3COOH
b) BrCH2COOH
c) ClCH2COOH
d) FCH2COOH
View Answer
Explanation: Presence of -I effect fluorine atom increases the acidic nature by withdrawing electrons.
F−CH2−COOH > Cl−CH2−COOH > Br−CH2−COOH > CH3COOH.
7. The reaction of HCOOH with conc.H2SO4 gives which of the following compound?
a) CO2
b) CO
c) Oxalic acid
d) Acetic acid
View Answer
8. Which one is strongest acid among following options?
a) CH2FCOOH
b) CH2ClCOOH
c) CHCl2COOH
d) CHF2COOH
View Answer
Explanation: CHF2−COOH. Difluoroacetic acid is strongest because presence of two F atoms increases its acidic nature.
9. Acetic acid is weak acid than sulphuric acid because which of the following reasons?
a) It decomposes on increasing temperature
b) It has less degree of ionisation
c) It has -COOH group
d) It has more inductive effect
View Answer
Explanation: CH3COOH is slightly ionised than H2SO4. Acetic acid is weak acid than sulphuric acid because It has less degree of ionization.
10. In CH3COOH and HCOOH, HCOOH will be which of the following?
a) Less acidic
b) Equally acidic
c) More acidic
d) cannot say about acidic behaviour
View Answer
Explanation: Presence of methyl group decreases the acidic character of acetic acid due to positive inductive effect (+I).
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all objective questions on Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs