This set of Engineering Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “E and Z Nomenclature of Geometric Isomers”.
1. What are Stereoisomers?
a) Isomers having a same molecular formula and same configuration
b) Isomers having a same molecular formula but different configuration
c) Isomers having a different molecular formula but the same configuration
d) Isomers having a different molecular formula and different configuration
View Answer
Explanation: Stereoisomerism is exhibited by isomers having the same molecular formula but different configuration.
2. What are diastereomers?
a) Molecules with non-superimposable mirror images
b) Molecules with superimposable mirror images
c) Molecules which do not have non-superimposable mirror images
d) None of the mentioned
View Answer
Explanation: Diastereomers are those stereomers which do not have non-superimposable mirror images.
3. Which type of compounds cannot exhibit geometrical isomerism?
a) Singly Bonded
b) Doubly Bonded
c) Triply Bonded
d) Cyclic Compounds
View Answer
Explanation: Triply bonded compounds cannot exhibit geometrical isomerism as the -C=C- bond in these molecules is linear.
4. Choose the correct option from the following.
a) A group gets priority if its atomic number is high
b) When atoms attached to a double bond have same atomic number, the first atoms are considered
c) A group gets priority if its atomic number is low
d) Lone pair gets more priority and is ranked above hydrogen
View Answer
Explanation: A group gets priority if its atomic number is high and When atoms attached to a double bond have same atomic number, the second atoms are considered.
5. In which type of projection we can get staggered and eclipsed conformations?
a) Newman Projection
b) Sawhorse Projection
c) Fischer Projection
d) Wedge Projection
View Answer
Explanation: A sawhorse projection can reveal staggered and eclipsed conformations.
6. Assign E or Z configuration to the given compound.
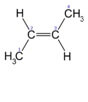
a) E-configuration
b) Z-configuration
c) S-configuration
d) R-configuration
View Answer
Explanation: Trans-2-butene has two geometric isomers and this is E-configuration.
7. Which of the following compounds have Z-configuration?
a) CH3CH3CH2>C=C<HCH3
b) HPh>C=C<COOHPh
c) H2NCl>C=C<COOHBr
d) CH3Ph>C=C<CH2CH3Br
View Answer
Explanation: All the other options are in E-configuration. Only the second option shows the Z-configuration.
8. Select the incorrect option from the following.
a) Fischer projections are two dimensional representations of three dimensional molecules
b) A molecule is achiral if it cannot be superimposed on its mirror reflection
c) E and Z notations are based on Cahn, Ingold and Prelog priority system
d) Chiral molecules which are non-superimposable mirror images of each other are enantiomers
View Answer
Explanation: A molecule is achiral if it is superimposable on its mirror reflection. All the other options are correct.
9. The cis-trans nomenclature is not applicable when _________
a) The different groups attached to the carbon atom of double bond are same
b) Atleast one of the group attached to each carbon atom is same
c) The different groups attached to the carbon atom of double bond are not same
d) The cis-trans nomenclature is applicable for all compounds
View Answer
Explanation: The cis-trans nomenclature is applicable when the different groups attached to the carbon atom of double bond are same or atleast one of the group attached to each carbon atom is same.
10. In glyceraldehydes, the complete sequence of priority is _________
a) –OH > -CH2OH > -CHO > -H
b) –H > -CH2OH > -CHO > -OH
c) –H > –OH > -CH2OH > -CHO
d) –OH > CHO > -CH2OH > -H
View Answer
Explanation: In glyceraldehydes, the complete sequence of priority is –OH > CHO > -CH2OH > -H.
Sanfoundry Global Education & Learning Series – Engineering Chemistry.
To practice all areas of Engineering Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Engineering Chemistry I Books
- Apply for 1st Year Engineering Internship
- Practice Engineering Chemistry II MCQ
