This set of Class 11 Chemistry Chapter 4 Multiple Choice Questions & Answers (MCQs) focuses on “Chemical Bonding and Molecular Structure”. These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation.
1. Atoms obtain octet configuration when linked with other atoms. This is said by _________
a) Lewis
b) Kossel
c) Langmuir
d) Sidgwick
View Answer
Explanation: The above statement says that the atoms achieve a stable octet configuration when joined with other atoms through chemical bonds as postulated by Lewis. An example of this is the formation of NaCl molecule where Na and Cl transfer electrons to each other forming Na+ and Cl–.
2. Find out the correct Lewis symbol for the atom carbon among the following options.
a) .C:
b) :C.
c) :C:
d) .C.
View Answer
Explanation: An American chemist G.N. Lewis created Lewis symbols as a notation to represent the valance electrons in an atom. As the carbon atom has 4 electrons in its outer shell, it is represented by 4 dots around it.
3. What’s the group valance of atoms in the halogen family?
a) 2
b) 1
c) 9
d) 7
View Answer
Explanation: The group valance can be calculated from Lewis symbols either by subtracting it from eight (more than 4) or having it equal (less than 4). The halogen family has 7 electrons in their outer orbit. So 8 – 7 = 1. Therefore the valency of the halogen family is 1.
4. Highly electropositive Alkali metals are separated from highly electronegative halogens by _________
a) noble gases
b) oxygen family
c) f-block elements
d) 7th period
View Answer
Explanation: According to Kossel, Highly electropositive Alkali metals are separated from highly electronegative halogens by noble gases. This is because Alkali metals are the 1st group and halogens the 17th group. Elements in 18th group i.e. nobles are preceded by group 17 elements and succeeded by group 1 elements.
5. Sharing or transfer of electrons from one atom to the other to attain stable octet configuration follows _______
a) Duet rule
b) Triplet rule
c) Octet rule
d) Septet rule
View Answer
Explanation: As per the electronic theory of chemical bond that’s put forth by Lewis &Kossel states that the atoms follow the octet rule by sharing or transfer of electrons from one atom to the other to attain stable octet configuration.
6. In the covalent bond, atoms share electrons to achieve octet configuration.
a) True
b) False
View Answer
Explanation: In the year 1919, Langmuir postulated the theory of covalent bond and its formation by combining with Lewis theory. An example of this is the formation of Cl2, Two atoms of Cl combine by sharing the 7th electron in its outer shell.
7. Which of the following molecule doesn’t involve covalent bond?
a) H2O
b) CCl4
c) NaCl
d) O2
View Answer
Explanation: The formation of NaCl molecule where Na and Cl transfer electrons to each other forming Na+ and Cl–. There is no sharing of electrons i.e. no covalent bond. Whereas the molecules H2O, Cl2 and O2 involve sharing of electrons.
8. Calculate the formal charge of C in CH4.
a) 4
b) 1
c) -4
d) 0
View Answer
Explanation: The formula for finding out the formula charge of an in a molecule = total number of valence electrons – total number of non-bonding electrons – 1/2(total number of bonding electrons). So here, formal charge of C = 4 – 0 – 8/2 = 0.
9. Which of the following doesn’t follow octet rule?
a) CH4
b) CCl4
c) HCl
d) NO2
View Answer
Explanation: Though octet rule is widely known, it does have a few limitations. The compound nitrogen dioxide NO2 doesn’t follow the octet rule. It’s a molecule with an odd number of electrons. Even the nitric oxide NO doesn’t follow.
10. Calculate the formal charge of the middle atom in the ozone molecule.
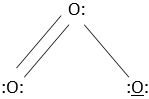
a) 1
b) -1
c) 0
d) -2
View Answer
Explanation: The formula for finding out the formula charge of an in a molecule = total number of valence electrons – total number of non-bonding electrons – 1/2 (total number of bonding electrons). So here, a formal charge of central O is 6 – 2 – 6/2 = 1.
More MCQs on Class 11 Chemistry Chapter 4:
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 2)
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 3)
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 4)
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 5)
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 6)
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 7)
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 8)
- Chapter 4 – Chemical Bonding and Molecular Structure MCQ (Set 9)
To practice all chapters and topics of class 11 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Class 11 - Chemistry Books
- Practice Class 11 - Mathematics MCQs
- Practice Class 11 - Physics MCQs
- Practice Class 11 - Biology MCQs
- Practice Class 12 - Chemistry MCQs
