This set of Organic Chemistry Question Bank focuses on “Benzene diazonium chloride – 2”.
1. Replacement of diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared which type of reaction?
a) Direct elimination
b) Addition reaction
c) Direct substitution
d) Replacement reaction
View Answer
Explanation: Replacement of diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by direct substitution.
2. What happens when benzene diazonium chloride is treated with potassium cyanide in presence of Cu powder?
a) Benzophenone
b) Methyl isocyanide
c) Acetonitrile
d) Benzonitrile
View Answer
Explanation: By treating diazonium salts with cuprous cyanide or KCN and copper powder it forms aryl nitrile. Illustrative is the preparation of benzonitrile using the reagent cuprous cyanide: C6H5N+2 + CuCN → C6H5CN + Cu+ + N2.
3. By treating diazonium salts with cuprous cyanide or KCN and copper powder it forms which of the following compound?
a) Citric acid
b) Benzoic acid
c) Aryl nitrile
d) Oxalic acid
View Answer
Explanation: By treating diazonium salts with cuprous cyanide or KCN and copper powder it forms aryl nitrile. The cyano group usually cannot be introduced by nucleophilic substitution of haloarenes, but such compounds can be easily prepared from diazonium salts.
4. Benzene diazonium chloride forms orange red dye with which of the following compound?
a) Nitrophenol
b) Benzophenol
c) Resorcinol
d) Methanol
View Answer
Explanation: Benzene diazonium chloride forms orange red dye with resorcinol, Reaction of benzene diazonium chloride with resocinol in basic medium is a coupling reaction,in which p-hydroxyazobenzene is obtained .Which is nothing but orange dye.
5. Which of the following is compound A for following sequence of reaction gave benzoic acid benzoic acid?
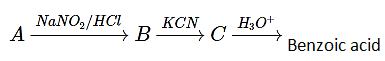
a) Nitrobenzene
b) Aniline
c) Benzaldehyde
d) Amides
View Answer
Explanation: In the following sequence reaction between aniline and NaNO2/HCl will form diazonium salt and with KCN it will form cyanide and at last carboxylic acid will be formed.
6. In the series of reaction, what are X and Y are respectively?
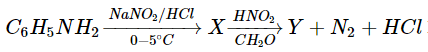
a) C6H5−N=N−C6H5, C6H5N+2Cl–
b) C6H5N+2Cl–, C6H5−N=N−C6H5
c) C6H5N+2Cl–, C6H5NO2
d) C6H5NO2, C6H6
View Answer
Explanation: In the following sequence reaction between aniline and NaNO2/HCl will form diazonium salt and with HNO2 nitrobenzene will be formed.
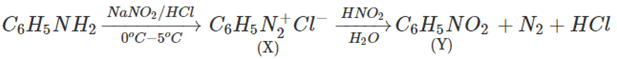
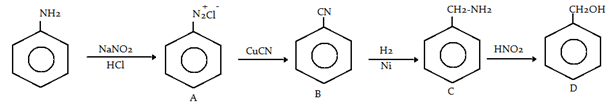
7. Aniline in a set of reactions yielded a product D. The structure of product D would be
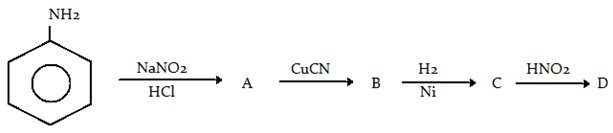
a) C6H5CH2NH2
b) C6H5NHCH2CH3
c) C6H5NHOH
d) C6H5CH2OH
View Answer
Explanation: First of all, diazonium will be formed and then it will show Sandmeyer reaction with CuCN and then reduction of cyanide and oxidation to benzyl alcohol.
8. Azo dye is prepared by the coupling of phenol and which of the following compound?
a) Diazonium chloride
b) o-nitro aniline
c) Benzoic acid
d) Chlorobenzene
View Answer
Explanation: The most widely practiced reaction of diazonium salts is azo coupling. In this process, the diazonium compound is attacked by, i.e., coupled to, electron-rich substrates. When the coupling partners are arenes such as anilines and phenols, the process is an example of electrophilic aromatic substitution.
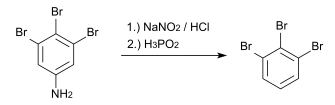
9. Identify the product in following order when 3,4,5-Tribromoaniline undergoes diazotisation followed by attack of H3PO2?
a) 3, 4,5-Tribromobenzene
b) 1, 2, 3-Tribromobenzene
c) 2, 4, 6-Tribromobenzene
d) 3, 4, 5-Tribromo nitro benzene
View Answer
Explanation: When 3,4,5-Tribromoaniline undergoes diazotisation followed by attack of H3PO2, 1, 2, 3-Tribromobenzene is formed.
10. When diazonium salt solution is treated with KI, it forms which of the following compound?
a) Bromobenzene
b) Iodobenzene
c) Phenol
d) Acid
View Answer
Explanation: Iodine is not easily introduced into the benzene ring directly. However, it can be introduced by treating aryldiazonium cations with potassium iodide: C6H5N+2 + KI → C6H5I + K+ + N2.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice Organic Chemistry Question Bank, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
