This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Acid Anhydrides”.
1. What is the major product of the following reaction?
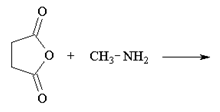
a) 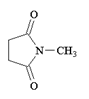
b) 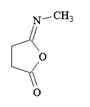
c) 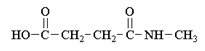
d) 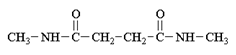
View Answer
Explanation: In order for this product to form, a second methyl amine nitrogen atom would have to attack the carbonyl carbon of the carboxylate anion (the leaving group of the original anhydride) and this is energetically not favoured due to repulsion by the negative charge on the oxygen atom.
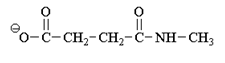
2. What will be the reactivity order of the following with water?
a) acid halide > ester > acid anhydride > amide
b) acid anhydride > amide > acid halide > ester
c) amide > ester > acid anhydride > acid halide
d) acid halide > acid anhydride > ester > amide
View Answer
Explanation: Acid halide are more reactive than anhydrides, which are more reactive than esters, which are more reactive than amides. This is due to the electronegative group, such as chlorine, polarizing the carbonyl group more strongly than an alkoxy group (ester) or an amino group (amide).
3. Which of the following is an anhydride?
a) 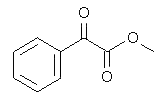
b) 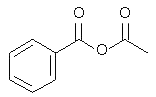
c) 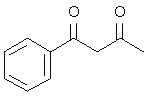
d) 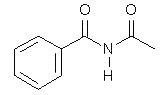
View Answer
Explanation: Acid anhydrides, or simply anhydrides, are compounds which would react additively with water to form two molecules of the parent carboxylic acid. (Mixed anhydrides, RCOOCOR’, i.e. anhydrides derived from two carboxylic acids would naturally re-form the two carboxylic acids from which they were obtained.)
4. Which of the following compounds is not an acid anhydride?
a) 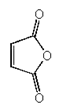
b) 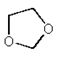
c) 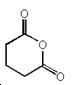
d) 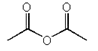
View Answer
Explanation: An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O.
5. Acetic anhydride is less reactive toward attack by a nucleophile than which of the following?
a) Acetic acid
b) Ethyl acetate
c) Acetyl chloride
d) Acetonitrile
View Answer
Explanation: Nucleophilic substitution reactions usually take place in two steps: addition of the nucleophile and elimination of a leaving group. Although both steps can affect the overall rate of the reaction, it is generally the first step that is rate-limiting. Therefore, any factor that makes the carbonyl group of the carboxylic acid derivative more easily attacked will favor the reaction.
6. Which of the following compounds would be converted to acetic anhydride when treated with sodium acetate?
a) Acetaldehyde
b) Acetyl chloride
c) Methyl acetate
d) Acetamide
View Answer
Explanation: The acid chloride can then be converted to a less reactive derivative such as an anhydride.
7. Which will be the product of the following reaction?
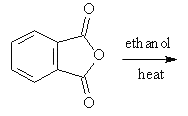
a) 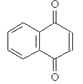
b) 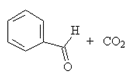
c) 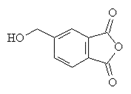
d) 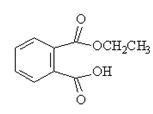
View Answer
Explanation: The product when reaction of acid anhydride with ethanol is the ester ethyl ethanoate, as shown in given reaction.
(CH3CO)20 + ROH → CH3COOR + CH3COOH
But, here the acid anhydride is closed so both ester and carboxylic part will be in same molecule.
8. Which of the following is most reactive?
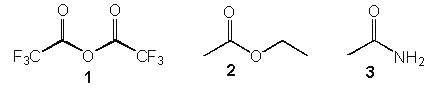
a) 1
b) 2
c) 3
d) 1 and 2
View Answer
Explanation: Acid anhydrides are more reactive than esters, which are more reactive than amides. This is due to the electronegative group, polarizing the carbonyl group more strongly than an alkoxy group (ester) or an amino group (amide).
9. What is the formed product when acid anhydride is hydrolyzed?
a) Aldehyde
b) Ketone
c) Alcohols
d) Carboxylic acid
View Answer
Explanation: Hydrolysis of acid anhydrides in water occurs at a slow rate and may also need heating (boiling) with water – a reaction rate which contrasts rather strongly with the acid halides and leads to formation of carboxylic acid.
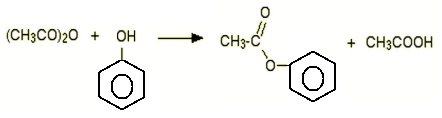
10. What will be the product for the following reaction?
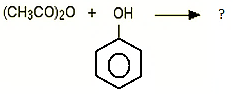
a) Ester and carboxylic acid
b) Ether and carboxylic acid
c) Ketones and ester
d) Aldehyde and carboxylic acid
View Answer
Explanation: Phenol and ethanoic anhydride reacts to form, phenyl ethanoate with ethanoic acid.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
