This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Reactions of Phenols”.
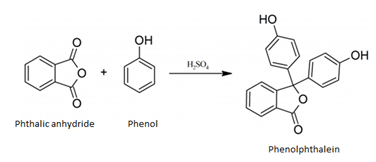
1. Phenolphthalein is obtained by heating phenol with conc. H2SO4 and which of the following reactant?
a) Benzyl alcohol
b) Benzene
c) Benzoic acid
d) Phthalic anhydride
View Answer
Explanation: Phenolphthalein is obtained by heating phenol with conc. H2SO4 and phthalic anhydride, as shown in the given reaction.
2. Salicylaldehyde can be prepared from which of the following reactants?
a) Phenol and chloroform
b) Phenol, chloroform and sodium hydroxide
c) Phenol, carbon tetrachloride and NaOH
d) Phenol, carbon tetrachloride
View Answer
Explanation: Salicylaldehyde is prepared from phenol and chloroform by heating with sodium hydroxide or potassium hydroxide in a Reimer–Tiemann reaction.
3. When phenol is treated with excess of bromine water, it gives which of the following product?
a) m-bromophenol
b) o-and p-bromophenol
c) 2,4-dibromophenol
d) 2,4,6-tribromophenol
View Answer
Explanation: In phenol, the hydroxyl group serves to greatly activate the benzene ring, significantly increasing its reactivity, hence susceptibility to substitution. The reaction occurs at room temp and even goes to maximum substitution of the 3 possible locations (2,4,6). result is 2,4,6-tribromophenol which is a white ppt.
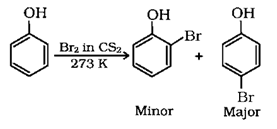
4. Phenol reacts with bromine in carbon disulphate at low temperature to give which of the following product?
a) m-bromophenol
b) o-and p-bromophenol
c) p-bromophenol
d) 2,4,6-tribromophenol
View Answer
Explanation: Phenol reacts with bromine in carbon disulphate at low temperature to give o-and p-bromophenol.
5. Bromine reacts with phenol and decolorize orange color and turns it to which of the colored precipitate?
a) white precipitate
b) pink precipitate
c) blue precipitate
d) black precipitate
View Answer
Explanation: Bromine reacts with phenol and decolorize orange color and turns it to white precipitate of brominated phenol.
6. Which of the following regents may be used to distinguish between phenol and benzoic acid?
a) Aqueous NaOH
b) Tollen’s reagent
c) Molisch reagent
d) Neutral FeCl3
View Answer
Explanation: Neutral FeCl3 test regents may be used to distinguish between phenol and benzoic acid as Phenol will give violet coloration but benzoic acid will not give coloration with neutral FeCl3.
7. What is the major product obtained on interaction of phenol with sodium hydroxide and carbon dioxide?
a) Benzoic acid
b) Salicyladehyde
c) Salicylic acid
d) Phthalic acid
View Answer
Explanation: Reacting the nucleophilic phenolate salt with carbon dioxide under high pressure / temperature results in regioselective ortho-substitution. This process is also known as the Kolbe-Schmitt synthesis. o-hydroxybenzoic acid is more commonly known as salicylic acid.
8. Picric acid is formed when phenol react with which of the following reactant?
a) Formaldehyde
b) Hydrogen
c) Nitric acid
d) Hydrochloric acid
View Answer
Explanation: Nowadays picric acid is prepared by treating phenol first with concentrated sulfuric acid which converts it to phenol-2,4-disulfuric acid and then with concentrated nitric acid to get 2,4,6-trinitrophenol.
9. Bakelite is formed when phenol react with which of the following reactant?
a) Formaldehyde
b) Hydrogen
c) Nitric acid
d) Sulphuric acid
View Answer
Explanation: Bakelite is a thermosetting polymer which is made by reaction between phenol and HCHO.
10. Phenol undergoes ionization to become more stable by reacting with which of the following chemical species?
a) negative ions
b) positive ions
c) radicals
d) neutral atoms
View Answer
Explanation: Phenol undergoes ionization to become more stable by reacting with negative ions as the negative charge is no longer entirely localised on the oxygen but is spread out around the whole ion. Spreading the charge around makes the ion more stable than it would be if all the charge remained on the oxygen.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
