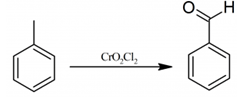This set of Advanced Organic Chemistry Questions and Answers focuses on “Preparation of Benzaldehyde & Aromatic Ketones”.
1. The oxidation of toluene to benzaldehyde by chromyl chloride is called as which of the following?
a) Cannizzaro reaction
b) Wurtz reaction
c) Etard reaction
d) Reimer-Tiemann reaction
View Answer
Explanation: The oxidation of toluene to benzaldehyde by chromyl chloride is called as Etard reaction.
2. Benzaldehyde can be prepared by oxidation of toluene by which of the following reagent?
a) Acidic KMnO4
b) K2Cr2O7
c) CrO2Cl2
d) basic KMnO4
View Answer
3. The oxidation of benzyl chloride with lead nitrate gives which of the following compound?
a) Benzaldehyde
b) Benzyl alcohol
c) Benzoic acid
d) p-chlorobenzaldehyde
View Answer
Explanation: The oxidation of benzyl chloride with lead nitrate gives benzaldehyde as shown in given reaction. Pb(NO3)2 is a mild oxidizing agent so benzaldehyde is formed during the oxidation of benzyl chloride with Pb(NO3)2.
4. Which of the following is the commercial method of preparation of benzaldehyde?
a) Oxidation of toluene
b) Oxidation of benzyl chloride
c) Oxidation of benzyl alcohol
d) Etard reaction
View Answer
Explanation: This involves the treatment of benzyl alcohol with dil. HNO3 or acidic potassium dichromate or chromic anhydride in acetic anhydride or with copper catalyst at 350o C. This process is used for commercial production of benzaldehyde.
5. Which of the following is the not a method of preparation of benzaldehyde?
a) Gattermann Koch synthesis
b) Etards reaction
c) Stephan’s reaction
d) Oxidation of secondary alcohol
View Answer
Explanation: Oxidation of secondary ketones is the method of formation of ketones not aldehydes.
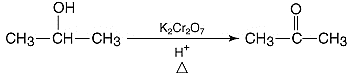
6. What reagents will be used in the preparation of benzaldehyde via Gattermann Koch synthesis?
a) Carbon dioxide and HCl
b) Carbon monoxide and HCl
c) Oxygen and H2SO4
d) Carbon monoxide and H2SO4
View Answer
Explanation: Benzene is converted into benzaldehyde by passing a mixture of carbon monoxide and HCl gas under high pressure into the ether solution of benzene in presence of anhydrous aluminum chloride and cuprous chloride.
7. Partial reduction of phenyl cyanide with stannous chloride and passing dry HCl gas in ether solution followed by hydrolysis of the aldimine stannic chloride with water to form benzaldehyde is called as which of the following method of preparation of benzaldehyde?
a) Gattermann Koch synthesis
b) Etards reaction
c) Stephan’s reaction
d) Gattermann reaction
View Answer
Explanation: Partial reduction of phenyl cyanide with stannous chloride and passing dry HCl gas in ether solution followed by hydrolysis of the aldimine stannic chloride with water to form benzaldehyde is called as Stephan’s reaction.
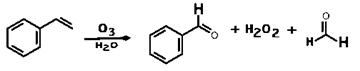
8. Ozonolysis of styrene will lead leads to formation of benzaldehyde along which compound?
a) O2
b) H2O2
c) HCHO
d) H2O2 and HCHO
View Answer
Explanation: Ozonolysis of styrene will lead leads to formation of benzaldehyde H2O2 and HCHO.
9. Which of the following cannot be used in formation of benzaldehyde by Grignard reagent?
a) HCN
b) Carbon monoxide
c) Ethyl format
d) HNC
View Answer
Explanation: Reagents like carbon monoxide or HCN and ethyl format can be used for formation of benzaldehyde by Grignard reagent.
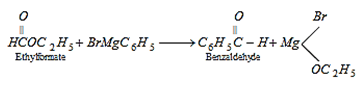
10. What is the laboratory method for the formation of benzaldehyde?
a) Gattermann Koch synthesis
b) Etards reaction
c) Stephan’s reaction
d) Oxidation of benzyl chloride
View Answer
Explanation: Benzaldehyde conveniently prepared by boiling benzyl chloride with copper nitrate or lead nitrate solution in a current of carbon dioxide.
11. Formation of aromatic ketone forms from benzene by CH3COCl?
a) Friedal craft alkylation
b) Friedal craft dealkylation
c) Friedal craft acylation
d) Friedal craft hydroxyalkylation
View Answer
Explanation: Friedal craft acylation froms aromatic ketone. A proper choice of acid chloride here gives the desired ketone. The method works for all types of ketones though the bulkier aryl acid chlorides may require stronger / longer heating.
12. What will be the product for the given reaction?
a) Propanone
b) Acetophenone
c) Diphenylmethanone
d) Aliphatic and aromatic
View Answer
Explanation: Heating solid calcium salts of benzoic acid with those of any other carboxylic acid, except methanoic acid, gives the ketones in low yields.
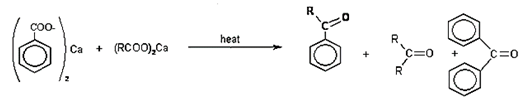
13. What will be the product of the given reaction?
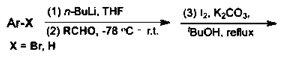
a) Propanone
b) Acetophenone
c) Diphenylmethanone
d) Aliphatic and aromatic
View Answer
Explanation: Aromatic ketones were efficiently prepared in good yields by the reactions of aryl bromides with n-BuLi, followed by the reactions with aromatic aldehydes or aliphatic aldehydes and the subsequent treatment with molecular iodine and K2CO3, in a one-pot method.
14. Which reactant can be used to form aromatic ketone for the following reaction?
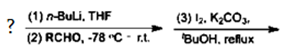
a) Arenes
b) Aldehyde
c) Alcohol
d) Terperens
View Answer
Explanation: Aromatic ketones were efficiently prepared in good yields by the reactions of arenes with n-BuLi, followed by the reactions with aromatic aldehydes or aliphatic aldehydes and the subsequent treatment with molecular iodine and K2CO3, in a one-pot method.
15. Aromatic ketones were synthesized from aromatic compounds via liquid‐phase oxidation at 60 °C and 1 atm over vanadium‐containing which catalyst?
a) MCM-41
b) MCM-48
c) ZSM-5
d) ZK-5
View Answer
Explanation: Aromatic ketones were synthesized from aromatic compounds via liquid‐phase oxidation at 60 °C and 1 atm over vanadium‐containing MCM‐41 catalysts using a batch reactor. The catalysts were prepared by direct hydrothermal (4V‐MCM‐41) and wet impregnation (9V/MCM‐41) methods.
16. Which of the following cannot be used as reactant in preparation of aromatic ketones from diazonium salt?
a) formaldoxime
b) Acetaldoxime
c) propionaldoxime
d) Terpernes
View Answer
Explanation: Diazonium salts react with formaldoxime, acetaldoxime, propionaldoxime, and acetaldehyde semicarbazone severally, under carefully controlled conditions, to give arylated derivatives which can be hydrolysed to aromatic ketones.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice advanced questions and answers on all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books