This set of Organic Chemistry Mcqs focuses on “Nuclear Magnetic Resonance – 2”.
1. In NMR spectroscopy, what is the product of Nuclear ‘g’ factor (gN), the nuclear magneton and the magnetic field strength (B0)?
a) energy of transition from alpha to beta state
b) chemical shift
c) spin-spin coupling constant
d) magnetogyric ratio
View Answer
Explanation: In NMR spectroscopy. the product of Nuclear ‘g’ factor (gN), the nuclear magneton (βN) and the magnetic field strength (B0) gives the energy of transition from alpha to beta state. As shown below:
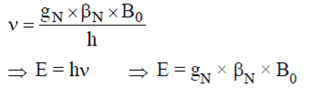
2. An organic compound having the molecular formulae C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR. What is the compound?
a) 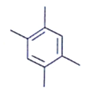
b) 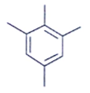
c) 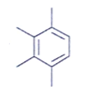
d) 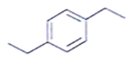
View Answer
Explanation: In compound a;
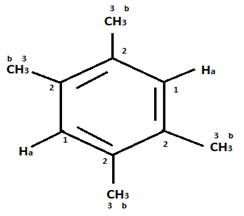
Here only 2 types of Hydrogen are present (two Ha & twelve Hb). So, 2 singlets only. There is no need to check 13C NMR. Still, if you check three types carbon are present (two C1, four C2 and four C2 carbon). Hence, there peak for 13C NMR.
3. The 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 exhibits two singlets of 1:1 intensity. What will be the molar ratio of acetone to dichloromethane in the solution?
a) 3:1
b) 1:3
c) 1:1
d) 1:2
View Answer
4. What will be the strength of coupling between geminal protons in the following molecules?

a) Decrease as the size of ring increase
b) Increase as the size of ring increase
c) Remains same
d) No relation between the size of the ring & coupling
View Answer
Explanation: Geminal proton 2J coupling i.e. coupling of H & H on same carbon.
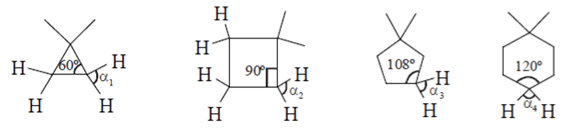
as ring size increases, bond angle outside decreases i.e. α1 > α2 > α3 > α4 (according % s character). If B.A. decreases, 2J coupling increases.
5. What is the value of gyramagnetic ratio of proton?
a) 41.10 radian/Tesla
b) 42.57 MHz/Tesla
c) 26.75 radian/Tesla
d) 41.10 MHz/Tesla
View Answer
Explanation:
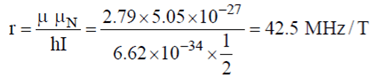
Where, I: nucleus spin=1/2
μn: nuclear magneton=5.05×10-27
μ: magnetic moment by protons=2.79
h: Plank constant= 6.63x 10-34joule-seconds.
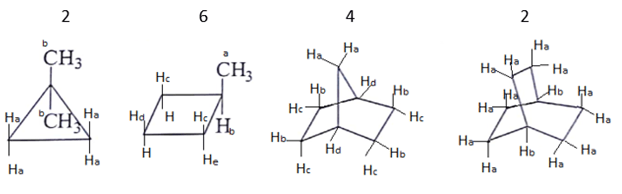
6. What are the number of signals in 1H NMR in the given molecules?
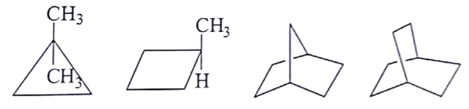
a) 3, 4, 4, 3 respectively
b) 2, 6, 4, 2 respectively
c) 2, 4, 6, 2 respectively
d) 2, 4, 2, 6 respectively
View Answer
Explanation: Different types of proton give the different type of signals, so the different hydrogen will give different numbers of signals.
As we can see first compound has two type of hydrogen Ha and Hb. Compound second has six type of hydrogen Ha, Hb, Hc, Hd, He and H. Compound third has four type of hydrogen Ha, Hb, Hc, Hd and l forth compound has two type of hydrogen Ha and Hb.
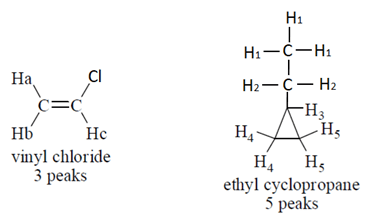
7. How many peaks are expected in low-resolution NMR spectrum of vinyl chloride and ethyl cyclopropane?
a) 3,5
b) 5,3
c) 6,3
d) 3,6
View Answer
Explanation: Vinyl chloride compound has three types of proton as shown below and ethyl cyclopropane has five types of protons so five peaks will be observed.
8. What will be the NMR frequency in MHz of bare 1H in a magnetic field of intensity 1.4092 tesla (given gN = 5.585 and μN = 5.05 x 10-27 JT-1)?
a) 60 MHz
b) 120 MHz
c) 100 MHz
d) 15 MHz
View Answer
Explanation: By using the below formula we can calculate NMR frequency, where h is Plank’s constant.
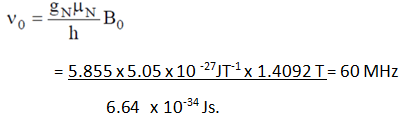
9. At room temperature, what is the number of singlet resonances observed in the 1H NMR spectrum of Me3CC(O)NMe2 (N, N-Dimethylpivalamide)?
a) 3
b) 4
c) 5
d) 2
View Answer
Explanation: As shown below; three signals are observed. 3Ha will experience shielding due to O–, so They are different from 3Hb.
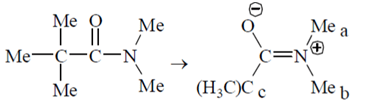
10. An organic compound (MF; C8H10O) exhibited the following 1H NMR special data: 62.5 (3H, s), 3.8 (314, s), 6.8 (2H, d, J 8 Hz), 7.2 (2H, d, J 8 Hz) ppm. What will be the compound among the choices?
a) 4-ethylphenol
b) 2-ethylphenol
c) 4-methylanisole
d) 4-methylbenzyl alcohol
View Answer
Explanation: C8H10O
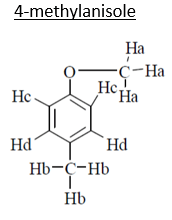
3Ha –> 3.8, singlet (deshielded because of –I of oxygen)
3Hb –> 2.5, singlet (No such –I)
2Hc –> 7.2, doublet (deshielded because of anisotropy effect of benzene as well as because of –I of oxygen)
2Hd –> 6.8, doublet (deshielded because of anisotropy of benzene).
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice MCQs on all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship

