This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Intramolecular Claisen Condensation”.
1. Which type of precursor is used as reactant in intramolecular Claisen condensation?
a) One molecule with an ester end
b) Two molecules of ester
c) One molecule with two ester ends
d) One molecule of ester and enolate
View Answer
Explanation: In intramolecular Claisen condensations, reactions occur for 1,6 and 1,7 diesters, as these substances result in the formation of compounds containing five and six membered rings, respectively.
2. Which is the main product of the following reaction?

a) 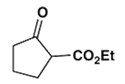
b) 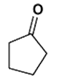
c) 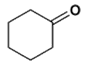
d) 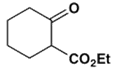
View Answer
Explanation: This is an example of an intramolecular Claisen condensation. These reactions occur for 1,6 and 1,7 diesters, as these substances result in the formation of compounds containing five and six membered rings, respectively.
3. What is the other name for the intramolecular Claisen condensation?
a) Perkin condensation
b) Stobbe condensation
c) Knoevenagel condensation
d) Dieckmann condensation
View Answer
Explanation: Diester compounds can be used to give an intramolecular Claisen condensation which is known as the Dieckmann condensation.
4. Dieckmann Condensation is intramolecular condensation of ______________ to form cyclic product.
a) diamide
b) diol
c) diester
d) diketone
View Answer
Explanation: These reactions occur for 1,6 and 1,7 diesters, as these substances result in the formation of compounds containing five and six membered rings.
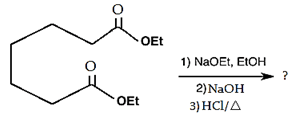
5. What will be the product of the following intramolecular Claisen condensation?
a) 
b) 
c) 
d) 
View Answer
Explanation: Favours the formation of the more stable 5- or 6-membered rings, as the ring stain in 5 and 6 membered rings are less.
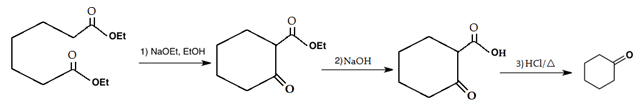
6. Which is the main product of the following intramolecular Claisen condensation?
a) 
b) 
c) 
d) 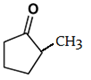
View Answer
Explanation: This reaction is an intramolecular Claisen condensation, which is followed by attack of carbocation on carbon attached to ester group.
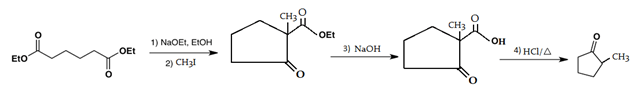
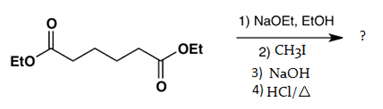
7. Which is the main product of the following intramolecular Claisen condensation?
a) 
b) 
c) 
d) 
View Answer
Explanation: This reaction is an intramolecular Claisen condensation, in which the product is a β-keto ester which gets deprotonated by the strong base in the reaction mixture and get converted into simple keto group.
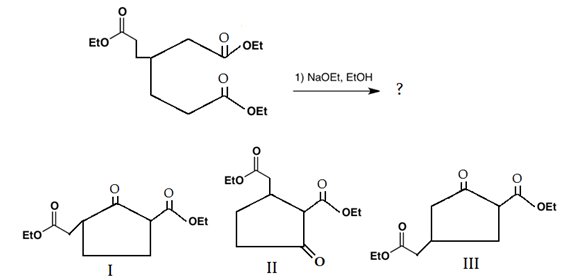
8. What will be the product(s) of the following intramolecular Claisen condensation?
a) I
b) II
c) I and II
d) II and III
View Answer
Explanation: This reaction is an intramolecular Claisen condensation, favours the formation of the more stable 5- or 6-membered rings, as the ring stain in 5 and 6 membered rings are less. In both II and III five membered ring is formed.
9. Which is the main product of the following intramolecular Claisen condensation?
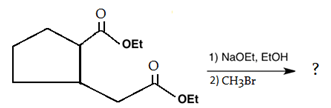
a) 
b) 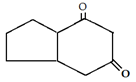
c) 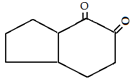
d) 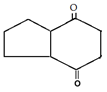
View Answer
Explanation: This reaction is an intramolecular Claisen condensation, favours the formation of the more stable 5- or 6-membered rings, as the ring stain in 5 and 6 membered rings are less and 6-membered rings are more stable than 5 membered.
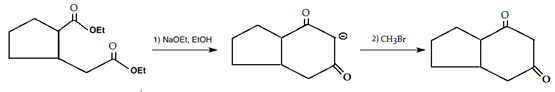
10. Which is the main product of the following intramolecular Claisen condensation?
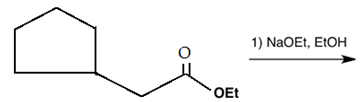
a) 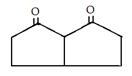
b) 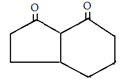
c) 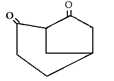
d) 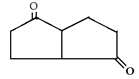
View Answer
Explanation: This reaction is an intramolecular Claisen condensation, favours the formation of the more stable 5- or 6-membered rings, as the ring stain in 5 and 6 membered rings are less and 6-membered rings are more stable than 5 membered. But in case bridge ring is present which is unstable as compared to 5 and 6 membered ring.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
