This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Vinylic Alcohol”.
1. Which is the vinylic alcohol position in the given diagram?
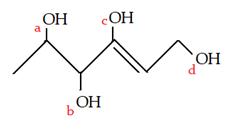
a) a
b) b
c) c
d) d
View Answer
Explanation: A vinyl halide is clearly a species with a formula H2C=C(OH)H, in which a halide is directly bound to an olefinic bond.
2. What is the IUPAC name for vinylic alcohol?
a) Ethanol
b) Methanol
c) Ethenol
d) Methenol
View Answer
Explanation: Vinyl alcohol, also called ethenol (IUPAC name), is the simplest enol. Vinyl alcohol or ethenol is CH₂=CH–OH. Ethanol has very ordinary, straightforward behavior, typical of an alcohol. Vinyl alcohol, however, because of its double bond (also referred to as its unsaturation), behaves considerably different.
3. Which of the following is not an isomer of vinylic alcohol?
a) Acetaldehyde
b) Ethylene oxide
c) Ethanol
d) Ethanal
View Answer
Explanation: With the formula CH2CHOH, vinyl alcohol is an isomer of acetaldehyde/ethanal and ethylene oxide. It is not an isomer of ethanol which has formula CH3CH2OH.
4. Which of the statement is not true about vinylic alcohols?
a) Several metal complexes are known that contain vinyl alcohol as a ligand
b) Vinyl alcohol was discovered in the molecular cloud Sagittarius B, using the 12-meter radio telescope
c) It can be formed by elimination of water from ethylene glycol at a temperature of 900 °C and low pressure
d) Vinylic alcohol shows no tautomerism
View Answer
Explanation: Under normal conditions, vinyl alcohol converts (tautomerizes) to acetaldehyde. At room temperature, acetaldehyde (H3CC(O)H) is more stable than vinyl alcohol (H2C=CHOH) by 42.7 kJ/mol. This tautomerization can be catalyzed via photochemical process.
5. Polyvinyl alcohol is soluble in which solvent?
a) protic
b) aprotic
c) polar aprotic
d) polar protic
View Answer
Explanation: Polyvinyl alcohol is a water-soluble synthetic polymer obtained by polymerisation of vinyl alcohol.
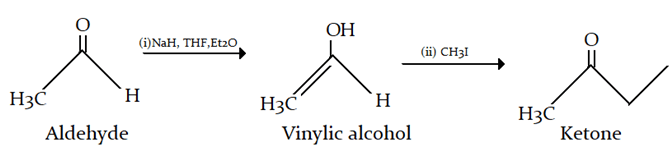
6. Which of the following set of reagents can not be used for the following conversion?
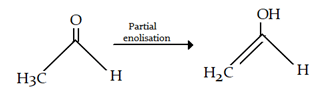
a) NaOH
b) R-OH
c) Net3
d) NaH
View Answer
Explanation: NaH is a strong base and will completely enolise keto form.
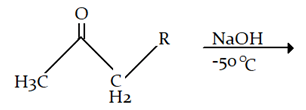
7. What will be the product for the given reaction?
a) 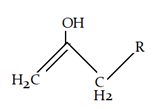
b) 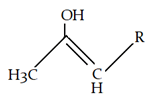
c) 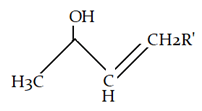
d) 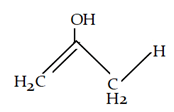
View Answer
Explanation: At low temperature hydrogen from adjacent carbonyl group will be removed instead of hydrogen from attached to carbon adjacent to alkyl group (R).
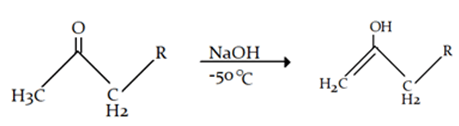
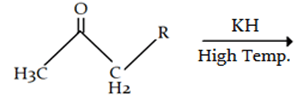
8. What will be the product for the given reaction?
a) 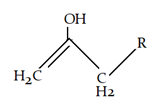
b) 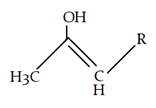
c) 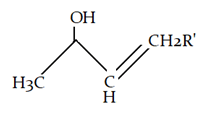
d) 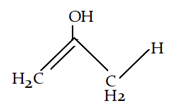
View Answer
Explanation: At high temperature hydrogen from attached to carbon adjacent to alkyl group (R) will be removed instead of hydrogen from adjacent carbonyl group will be removed.
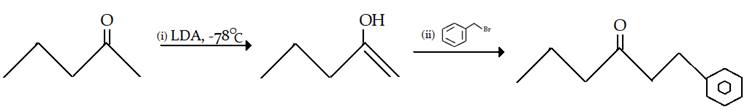
9. What is the final product for the given reaction?
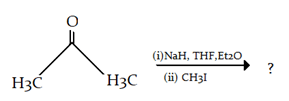
a) 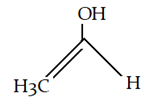
b) 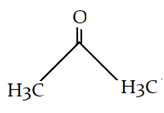
c) 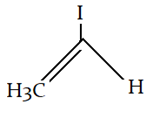
d) 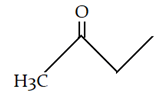
View Answer
Explanation: This is alkylation of carbonyl compound, via formation of enol form (vinylic alcohol), as shown in the given reaction.
10. What is the final product for the given reaction?
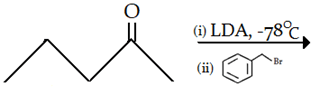
a) 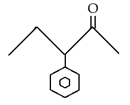
b) 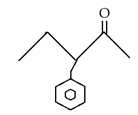
c) 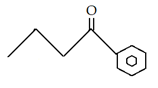
d) 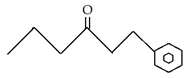
View Answer
Explanation: This is alkylation of carbonyl compound, via formation of enol form (vinylic alcohol), as shown in the given reaction.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
