This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Diastereomers”.
1. Which of the following is the definition of a pair of diastereomers?
a) A pair of stereoisomers each of which has two chirality centers
b) Any pair of stereoisomers
c) A pair of stereoisomers that are not mirror images of one another
d) A pair of stereoisomers that are non-superimposable mirror images of one another
View Answer
Explanation: Not all stereoisomers are diastereomers. For example, a pair of molecules which are non-superimposable mirror images of one another are enantiomers, not diastereomers.
2. What is the relationship between given compound?
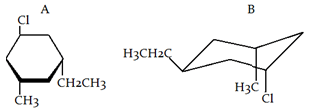
a) Diastereomer
b) Enantiomer
c) Identical
d) Don’t have any relation
View Answer
Explanation: Both molecules have the same molecular formula (C9H17Cl) and the same connectivity. Each molecule also has three stereocenters and does not contain a plane of symmetry. Therefore, both molecules are chiral. It can be determined that these molecules are non-superimposable and are not mirror images. Therefore, the molecules are diastereomers.
3. Which of the following is the diastereomer of the following compound?

a) 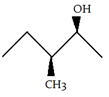
b) 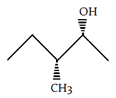
c) 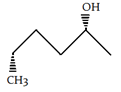
d) 
View Answer
Explanation: Both molecules are chiral. It can be determined that these molecules are non-superimposable and are not mirror images. Therefore, the molecules are diastereomers.
4. Which of the following can exist as diastereomers?
a) Lactic acid
b) 1-Butene
c) 2-Butene
d) Ethane
View Answer
Explanation: The two possible geometries of 2-butene are cis-2-butene and trans-2-butene; cis indicates that substituents are arranged on the same side of the double bond, while trans indicates opposite sides. These are stereoisomers; the atoms are connected in the same way but arranged differently in space.
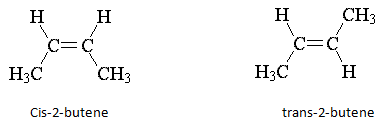
5. Which structure(s) represent(s) diastereomer(s) of the following compound?
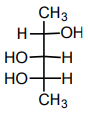
a) 
b) 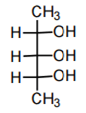
c) 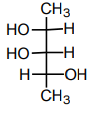
d) 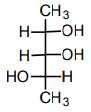
View Answer
Explanation: It can be determined that these molecules are non-superimposable and are not mirror images. Therefore, the molecules are diastereomers.
6. What is the relation between the given compound?
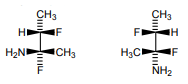
a) constitutional isomers
b) enantiomers
c) diastereomers
d) identical
View Answer
Explanation: It can be determined that these molecules are non-superimposable and are not mirror images. Therefore, the molecules are diastereomers.
7. What is the relation between the given compound?
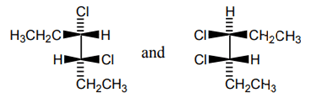
a) constitutional isomers
b) enantiomers
c) diastereomers
d) identical
View Answer
Explanation: It can be determined that these molecules are non-superimposable and are not mirror images. Therefore, the molecules are diastereomers.
8. What is the relation between the given compound?
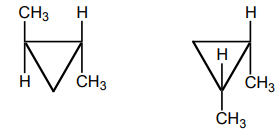
a) diastereomers
b) constitutional isomers
c) enantiomers
d) identical
View Answer
Explanation: It can be determined that these molecules are non-superimposable and are not mirror images. Therefore, the molecules are diastereomers.
9. What is the relation between the given compound?
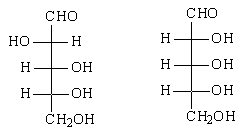
a) diastereomers
b) constitutional isomers
c) enantiomers
d) identical
View Answer
Explanation: It can be determined that these molecules are non-superimposable and are not mirror images. Therefore, the molecules are diastereomers.
10. Which of the following compounds would be suitable for preparing the necessary diastereomeric salts in preparation of Naproxen?
a) Quinoline
b) Tartaric acid
c) Malic acid
d) Cinchonidine
View Answer
Explanation: The correct answer is cinchonidine. Because naproxen contains a carboxylic acid, it will be necessary to use an amine to form a salt. This rules out malic acid and tartaric acid. The amine also has to be chiral in order to make the diastereomeric salt, so quinoline which is achiral.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
