This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Stereochemistry”.
1. Compounds which have different arrangements of atoms in space while having same atoms bonded to each other are said to have
a) position isomerism
b) functional group isomerism
c) chain isomerism
d) stereoisomerism
View Answer
Explanation: Stereoisomer his contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
2. Which of the following can make difference in optical isomers?
a) heat
b) temperature
c) polarized light
d) pressure
View Answer
Explanation: An optically active substance is one which can rotate the plane of polarisation of plane polarised light. if you shine a beam of polarised monochromatic light (light of only a single frequency – in other words a single colour) through a solution of an optically active substance, when the light emerges, its plane of polarisation is found to have rotated.
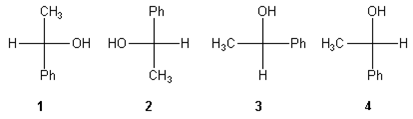
3. Which of the following Fischer projections is different from the other three?
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: When comparing Fischer projections, it is important to recognize that the horizontal bonds point out of the page, and vertical bonds point into the page. If a substituent is held in place, and the other three substituents are rotated, the new structure is the same as the original structure. Holding the phenyl substituent of 4 in place and rotating the other three clockwise gives 1, so these structures are the same.
4. Which of the following terms best describes the following pair of molecules?
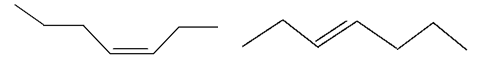
a) Isomers
b) Constitutional isomers
c) Configurational isomers
d) Geometrical isomers
View Answer
Explanation: These molecules have the same molecular formula (C7H14), making them isomers. However, the molecules differ in their spatial orientations due to a double bond. Therefore, the molecules can best be described as geometric isomers.
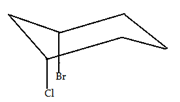
5. What is the relationship between the two groups in the following molecules?
a) They are equatorial to one another
b) They are axial to one another
c) They are cis to one another
d) They are trans to one another
View Answer
Explanation: Axial and equatorial are terms that describe the orientation of a single group with respect to a chair and can therefore be ruled out. The bromine and chlorine are both oriented downward, on the same side of the chair. By definition, they are cis to one another.
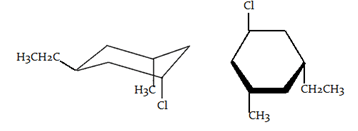
6. What is the stereochemical relationship between the following two molecules?
a) Geometrical isomers
b) Enantiomers
c) Diastereomers
d) Identical
View Answer
Explanation: Both molecules have the same molecular formula (C9H16BrCl) and the same connectivity. Each molecule also has three stereocenters, marked above with an asterisk, and does not contain a plane of symmetry. Therefore, both molecules are chiral. Using one of the methods outlined in this tutorial, it can be determined that these molecules are superimposable. Therefore, the molecules are identical.
7. Which of the following is an alkane which can exhibit optical activity?
a) Neopentane
b) Isopentane
c) 3–Methylpentane
d) 3–Methylhexane
View Answer
8. What is the molecular formula for the alkane of smallest molecular weight which possesses a stereogenic center?
a) C4H10
b) C5H12
c) C6H14
d) C7H16
View Answer
Explanation: See as the compounds who shows optical isomerism have a chiral carbon so an unsymmetrical alkane can show the optical isomerism. 6 carbon and 8 carbon alkane is not the answer. So, the smallest alkane is 7 carbon alkane is 3 methyl hexane which have 3rd carbon as chiral carbon.
9. Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?
a) Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixture
b) The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical properties
c) Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexane
d) The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvable
View Answer
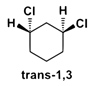
Explanation: In the below given diagram of trans-1,3-dichlorohexane the chlorines cannot be positioned diaxial or diequatorial but can only be axial and equatorial or vice versa. Additionally, if they were diastereomers or structural isomers, then they could be separated by physical means.
10. Which of the following statements can be deduced about the stereochemistry of this compound?
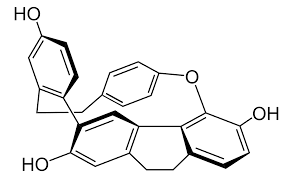
a) This compound is optically active because it has stereogenic centers that create cis-trans isomers
b) This compound is optically active because the compound contains a center, plane, or axis of chirality
c) This compound is not optically active since there are no stereogenic centers
d) This compound is not optically active because of its symmetry
View Answer
Explanation: In identifying stereogenic centers, any quaternary or tertiary carbons would be a good place to look. There are no quaternary carbons, and all the tertiary carbons are part of a benzene ring structure.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship

