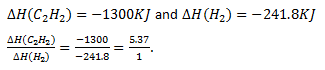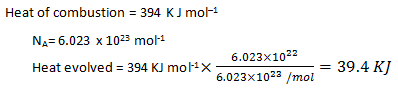This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Combustion of Organic Compounds”.
1. What is the concentration of carbon dioxide in the atmosphere?
a) 3.5 x 106 ppm
b) 1.0 x 102 ppm
c) 1.6 x 105 ppm
d) 1.0 x 103 ppm
View Answer
Explanation: The concentration of carbon dioxide is 3.5 x 106 ppm, that makes it sixth abundant molecule in atmosphere.
2. Which of the following reaction has a faster rate of reaction for nascent oxygen atom?
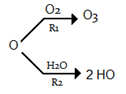
a) R1 >> R2
b) R1 > R2
c) R1 << R2
d) R1 < R2
View Answer
Explanation: R1 = k1[O2] [O] and R2 = k2[H2O] [O].
In the stratosphere, oxygen atom most often reacts with molecular oxygen to regenerate ozone. The troposphere, unlike the stratosphere, contains considerable concentrations of water vapor and water reacts faster with O than does O2.
3. Which of the following is not true about combustion of hydrocarbons?
a) All hydrocarbons react in air to form carbon monoxide and then carbon dioxide
b) First step is always the reaction between the hydrocarbon and hydroxyl radical
c) Alkanes, the hydroxyl radical abstracts a hydrogen atom and forms a carbanion
d) With alkenes and alkynes, the electron-deficient hydroxyl radical adds to the multiple bond
View Answer
Explanation: With alkanes, the hydroxyl radical abstracts a hydrogen atom and forms a carbon-centered radical.
4. What is the type of reaction between alkanes and hydroxyl radical?
a) endothermic
b) exothermic
c) isothermal
d) isochoric
View Answer
Explanation: Because an O-H bond is stronger than a C-H, this step is exothermic.
CH3 CH3 + OH → CH3 CH2 + H.
5. Which of the following statement is incorrect about oxidation number?
a) Show electron density and about the tendency of an atom or molecule to engage oxidation-reduction reactions
b) The oxidation number is always given in Roman numerals while the formal charge is always given in numbers
c) To calculate an oxidation number, it is essential to know which element in a chemical bond is the most electronegative
d) It cannot be a negative number
View Answer
Explanation: Oxidation numbers are positive or negative numbers, but don’t confuse them with positive or negative charges on ions or valences.
6. With respect to enthalpy of combustion which of the following is correct?
a) ∆Hrxn = Σ∆Hf (product) – Σ∆Hf (reactant)
b) ∆Hrxn = Σ∆Hf (reactant) – Σ∆Hf (product)
c) ∆Hrxn = Σ∆Hf (product) + Σ∆Hf (reactant)
d) ∆Hrxn = 2 Σ∆Hf (product) – Σ∆Hf (reactant)
View Answer
Explanation: According to the definition of the enthalpy of combustion we compute the enthalpy of the reaction. Because the enthalpy is a thermodynamic state function, it doesn’t depend on the pathway.
So, ∆Hrxn = Σ∆Hf (product) – Σ∆Hf (reactant).
7. Equal volumes of C2H2 & H2 are combusted under identical condition. What will be the ratio of heat evolved for C2H2 and H2?
H2 (g) + 1/2 O2(g) → H2O(g), ∆H=-241.8 KJ
C2H2 (g) + 5/2 O2(g) → CO2(g) + H2O(g), ∆H=-1300 KJ
a) 5.37/1
b) 1/5.37
c) 1/1
d) 2.8/6.1
View Answer
8. The heat of combustion of carbon is 394 KJ/mol. What will be the heat evolved in combustion of 6.023 x 1022 atoms of carbon?
a) 64.7 KJ
b) 39.4 KJ
c) 42.4 KJ
d) 91.6 KJ
View Answer
9. The value ∆H transition of C (graphite) → C (diamond) is 1.9 kJ/mol at 25℃ entropy of graphite is higher than entropy of diamond. This implies that?
a) C(diamond) is more thermodynamically stable then C (graphite) at 25℃
b) C(graphite) is more thermodynamically stable than C (diamond) at 25℃
c) diamond will provide more heat on complete combustion at 25℃
d) ∆Gtransition of C (diamond) → C (graphite) is -ve
View Answer
Explanation: C(graphite) → C(diamond) trans ∆H = 1.9 kJ /mole
Sgraphite > Sdiamond
As, Sgraphite is higher so C (graphite) is more stable.
Diamond will provide more heat on complete combustion because it is more stable and have 3–D structure.
C diamond → Cgraphite
∆Gtransition = -ve then process is spontaneous.
10. Which of the following is the slow, low-temperature, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel?
a) Rapid combustion
b) Turbulent combustion
c) Spontaneous combustion
d) Smouldering
View Answer
Explanation: Smouldering occurs on the surface of the solid rather than in the gas phase. Smouldering is a surface phenomenon but can propagate to the interior of a porous fuel if it is permeable to flow.
The characteristic temperature and heat released during smouldering are low compared to those in the flaming combustion.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books