This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Chemical Properties of Aldehydes”.
1. During a reaction of Tollens test, the formation of mirror inside the tube is due to which of the following?
a) silver ions
b) silver atoms
c) silver compounds
d) silver nitrate
View Answer
Explanation: Tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid.
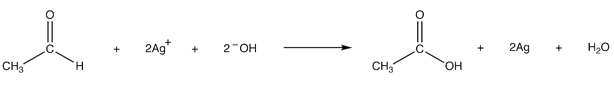
The reaction is accompanied by the reduction of silver ions in Tollens’ reagent into metallic silver, which, if the test is carried out in a clean glass test tube, forms a mirror on the test tube.
2. Reduction of Aldehydes to hydrocarbon take place in the presence of which of the following?
a) Zn amalgam and HCl acid
b) Pd/BaSO4
c) Anhydrous AlCl3
d) Ni/Pt
View Answer
Explanation: Reduction of Aldehydes to hydrocarbon take place in the presence of Zn amalgam and HCl acid, is a clemmensen reduction.
3. For C6H5CHO which of the following is incorrect?
a) On oxidation it yields benzoic acid
b) It is used in perfumery
c) It is an aromatic aldehyde
d) On reduction yields phenol
View Answer
4. Which of the following compound will undergo self-aldol condensation in the presence of cold dilute alkali?
a) C6H5CHO
b) CH3CH2CHO
c) CH≡C−CHO
d) CH2=CH−CHO
View Answer
Explanation: Carbon−carbon bond formation using strong and weak anion-exchange resins as green catalysts for self- and cross-aldol condensation of propanal in aqueous media was investigated. The reaction pathway followed the route of aldol condensation to a β-hydroxy aldehyde and dehydration to an α,β-unsaturated aldehyde. The resulting products were further converted to hemi-acetal.
5. Which of the following will not undergo aldol condensation?
a) Acetaldehyde
b) Propanaldehyde
c) Benzaldehyde
d) Trideuteroacetaldehyde
View Answer
Explanation: Deuterium behaves like H and hence trideuteroacetaldehyde also undergoes aldol condensation but benzaldehyde does not since it has no a-hydrogen.
6. Acetaldehyde cannot show which of the following test?
a) Iodoform test
b) Lucas test
c) Benedict’s test
d) Tollen’s test
View Answer
Explanation: Lucas reagent is a solution of anhydrous ZnCl2 & concentrated HCl. This solution is used to classify alcohols of low molecular weight. This reaction is substitution in which a chloride replaces hydroxyl group. It is to differentiate between Primary, Secondary & tertiary alcohols.
ROH + HCl → RCl + H2O
7. What will be the product if aldehyde reacts with NaOH?
a) Benzyl alcohol
b) Benzoic alcohol
c) Hydrobenzamide
d) Cinnamic acid
View Answer
Explanation: The Grignard reagent adds across the carbon-oxygen double bond:
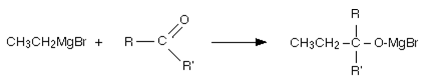
Dilute acid is then added to this to hydrolyse it, an alcohol is formed. One of the key uses of Grignard reagents is the ability to make complicated alcohols easily.
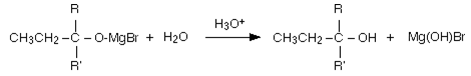
8. To distinguish between formaldehyde and acetaldehyde, we require which of the following reagent?
a) Tollen’s reagent
b) Fehling’s solution
c) Schiff’s reagent
d) Caustic soda solution
View Answer
Explanation: Reaction of formaldehyde is a Cannizzaro reaction when react with Dil. NaOH and reaction of acetaldehyde is aldol condensation reaction when react with Dil. NaOH.
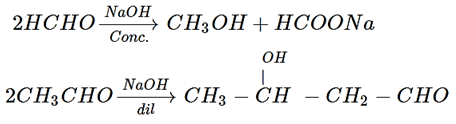
9. If formaldehyde and KOH are heated, then we get which of the following compound?
a) Acetylene
b) Methane
c) Methyl alcohol
d) Ethyl formate
View Answer
10. Acetaldehyde reacts with C2H5MgCl the final product is which of the following?
a) An aldehyde
b) A ketone
c) A primary alcohol
d) A secondary alcohol
View Answer
Explanation: One of the R groups is hydrogen and the other CH3 in ethanal. So, the final product has one CH3 group and one hydrogen attached:
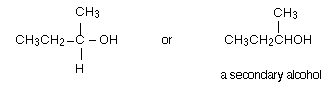
A secondary alcohol has two alkyl groups (the same or different) attached to the carbon with the -OH group on it.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs

