This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Chirality”.
1. Which statement about a chiral compound A is incorrect?
a) A racemate contains equal amounts of (+)-A and (–)-A
b) If A is resolved, it is separated into its enantiomers
c) (+)-A can also be labelled R-A, because (+) means the same as R
d) (+)-A and (–)-A will rotate polarized light equally but in opposite directions
View Answer
Explanation: Follow the direction of the remaining 3 priorities from highest to lowest priority (lowest to highest number, 1<2<3). A clockwise direction is an R (rectus, Latin for right) configuration. A dextrorotary compound is often prefixed “(+)-” or “d-“
2. Which of the following is the definition of chirality?
a) The superimposability of an object on its mirror image
b) A molecule with a mirror image
c) The non-superimposability an object on its mirror image
d) A molecule that has a carbon atom with four different substituents
View Answer
Explanation: Chirality is not restricted to molecules. While it is common for the presence of a carbon atom in a molecule with four different substituents to be the origin of chirality of a compound, it is not a requirement for chirality.
3. Which of the following compound(s) is/are chiral?
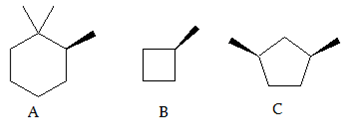
a) Only A and B
b) Only B
c) Only B and C
d) Only A
View Answer
Explanation: Chiral molecules are non-superimposable on their mirror images. A good way to determine whether a molecule is chiral is to determine whether it has a plane of symmetry. A molecule with a plane of symmetry is achiral (not chiral). Molecule 1 does not have a plane of symmetry so it is chiral. Molecules 2 and 3 both have planes of symmetry, so they are not chiral.
4. Which symmetry element makes the given compound achiral?

a) Plane of symmetry (POS)
b) Center of symmetry (COS)
c) Axis of symmetry (AOS)
d) Alternating axis of symmetry (AAOS)
View Answer
Explanation: In order for a molecule to be achiral, it must be superimposable on its mirror image. Molecules with a plane of symmetry or a center of symmetry meet this criterion.
5. Which of the following compound(s) is/are chiral?
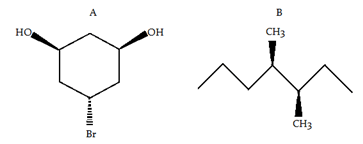
a) Both A and B
b) Only B
c) Only A
d) Neither A nor B
View Answer
Explanation: In order for a molecule to be chiral, it must not be superimposable on its mirror image. Molecules with a plane of symmetry or a center of symmetry do not meet this criterion. Molecule A has a plane of symmetry (shown above in fuchsia), molecule B has no symmetry.
6. The given compound is a chiral molecule.
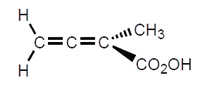
a) True
b) False
View Answer
Explanation: In order for a molecule to be chiral, it must not be superimposable on its mirror image. Molecules with a plane of symmetry or a center of symmetry do not meet this criterion. This molecule is having plane of symmetry, it is an achiral molecule.
7. Which symmetry element makes the given compound achiral?

a) Plane of symmetry (POS)
b) Center of symmetry (COS)
c) Axis of symmetry (AOS)
d) Alternating axis of symmetry (AAOS)
View Answer
Explanation: In order for a molecule to be achiral, it must be superimposable on its mirror image. Molecules with a center of symmetry meet this criterion.
8. The given compound is a chiral molecule.
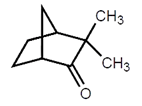
a) True
b) False
View Answer
Explanation: In order for a molecule to be chiral, it must not be superimposable on its mirror image. Molecules with a plane of symmetry or a center of symmetry do not meet this criterion. This molecule is not having any symmetry element, it is a chiral molecule.
9. Which of the following compound(s) is/are achiral?

a) I
b) II
c) III
d) I and II
View Answer
Explanation: In order for a molecule to be chiral, it must not be superimposable on its mirror image. Molecules with a plane of symmetry or a center of symmetry do not meet this criterion. Molecule II and III are not having symmetry element, they are chiral molecule. Molecule ‘I’ is having plane of symmetry, it is an achiral atom.
10. Which of the following compound(s) is/are chiral?
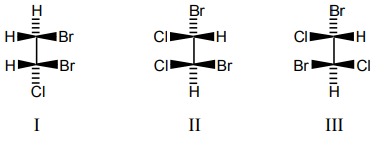
a) I
b) II
c) III
d) I and II
View Answer
Explanation: In order for a molecule to be chiral, it must not be superimposable on its mirror image. Molecules with a plane of symmetry or a center of symmetry do not meet this criterion. Molecule I and II are not having plane of symmetry, they are chiral molecule. Molecule ‘III’ is having plane of symmetry, it is an achiral atom.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
